Baloxavir marboxil
- CAS NO.:1985606-14-1
- Empirical Formula: C27H23F2N3O7S
- Molecular Weight: 571.55
- MDL number: MFCD31619272
- EINECS: 606-227-7
- Update Date: 2024-11-19 23:02:33
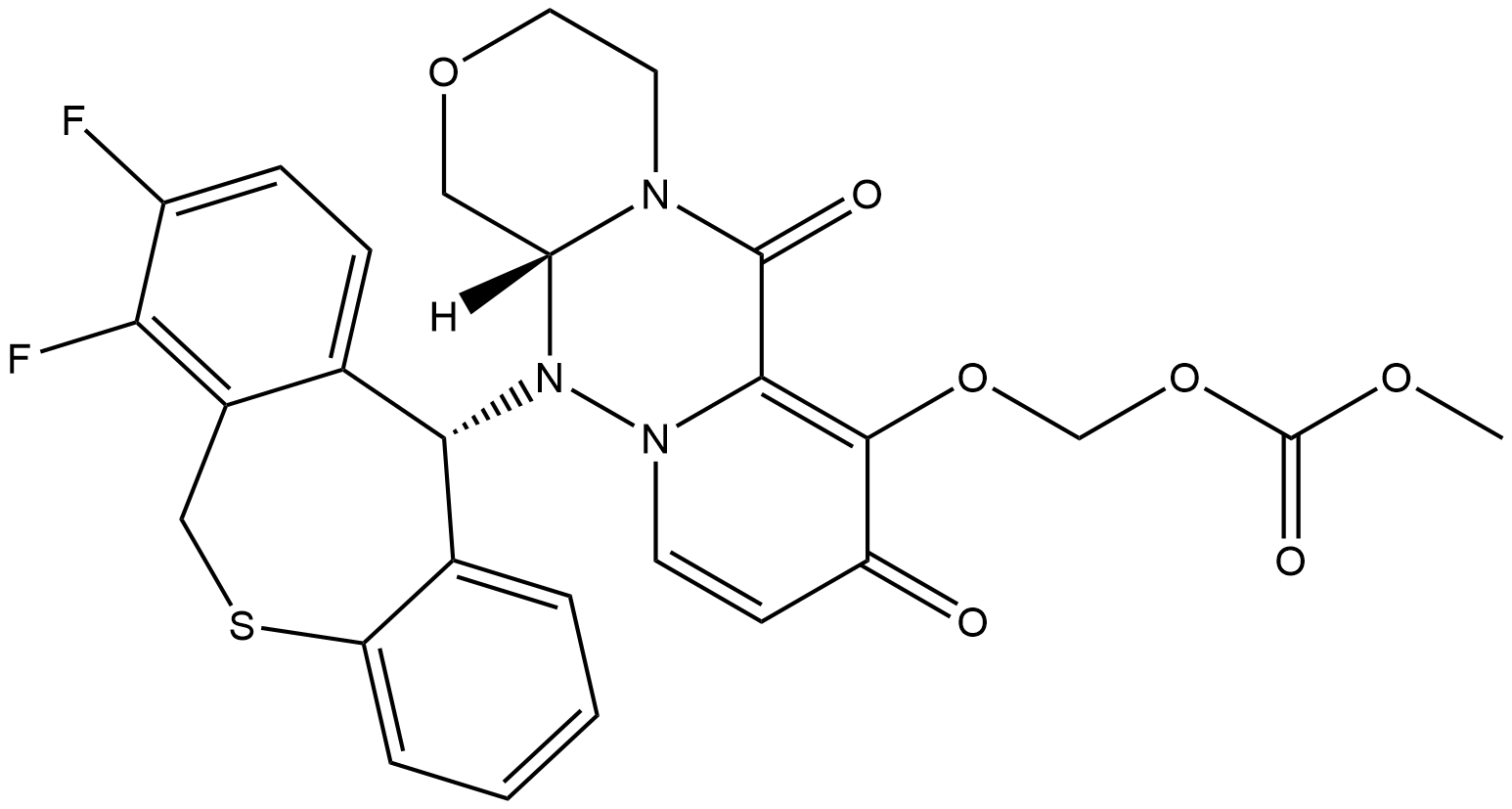
What is Baloxavir marboxil?
Absorption
Following oral administration of 40 mg baloxavir marboxil in adolescents and adults aged 12 years and older, the AUC was 5520 ng x hr/mL and the Cmax was 68.9 ng/mL. Following a 80 mg dose, the the AUC was 6930 ng x hr/mL and the Cmax was 82.5 ng/mL. The Tmax is about four hours. Food decreased Cmax by 48% and AUC0-inf by 36%.
In pediatric patients aged five to 12 years of age weighing less than 20 kg, the AUCinf was 5830 ng x hr/mL and the Cmax was 148 ng/mL following a 2 mg/kg dose. The AUCinf was 4360 ng x hr/mL and the Cmax was 81.1 ng/mL following a 40 mg dose in pediatric patients who weigh greater than or equal to 20 kg. The Tmax ranged from 3.5 to 4.5 hours.
Toxicity
Oral LD50 is >2000 mg/kg in rats.
There is limited clinical experience with baloxavir overdose. In one ascending single-dose study involving healthy volunteers, up to 80 mg dose of baloxavir was administered without notable safety concerns. Treatment of an overdose of baloxavir marboxil should consist of general supportive measures, including monitoring of vital signs and observation of the clinical status of the patient. There is no specific antidote for overdose with baloxavir marboxil. Baloxavir, the active ingredient, is unlikely to be significantly removed by dialysis due to high serum protein binding.
The Uses of Baloxavir marboxil
Baloxavir Marboxil is a prodrug of S-033447, which is an inhibitor of the cap-dependent endonuclease of influenza A and B viruses.
Background
Baloxavir marboxil is a novel cap-dependent endonuclease inhibitor that blocks influenza virus proliferation. It is a prodrug of baloxavir that is better absorbed than its active metabolite due to the addition of a phenolic hydroxyl group to its structure. The drug was initially approved in Japan in 2018, followed by approvals in the United States and the European Commission in 2018 and 2021, respectively.
Indications
Baloxavir marboxil is approved for treating acute, uncomplicated influenza in healthy adults, children over five, and high-risk individuals, within 48 hours of symptoms onset. It also serves as post-exposure prophylaxis for influenza in those over five. Caution is advised due to potential efficacy loss from viral changes.
Preparation
The synthesis of baloxavir marboxil involved the following steps: The coupling of the 1-R and 2 fragments was carried out under dehydration conditions of 1-propanephosphonic anhydride (T3P) and methanesulfonic acid at 70 °C to obtain protected baloxavir 19. Compound 19 was then reacted with 0.6 equivalents of PhBr and K2CO3. Debenzylation was then carried out using LiCl in CH3CONMe2 to give baloxavir acid in 94% yield. In the final step for the preparation of the prodrug, baloxavir acid was reacted with chloromethyl methyl carbonate in dimethylacetamide in 93% yield to form baloxavir marboxyl. 
The reaction mechanism for the final step for the synthesis of baloxavir marboxil(BXM).
Dosage
Influenza A and B
Adult: Acute uncomplicated influenza: 40-<80 kg: 40 mg; z80 kg: 80 mg (Oral).
Given as single dose, initiate within 48 hours after the onset of symptoms.
Child: Acute uncomplicated influenza: 2 12 years Same as adult dose (Oral).
brand name
XOFLUZA? (baloxavir marboxil)
Administration
Baloxavir marboxil can be administered irrespective of food intake but should be avoided with dairy, calcium-fortified drinks, polyvalent cation-containing laxatives, antacids, or supplements with calcium, iron, magnesium, selenium, or zinc to prevent interactions.
Biological Activity
Baloxavir marboxil is a prodrug that is metabolised to the active form baloxavir acid also known as S-033447. S-033447 is a small molecule inhibitor of the cap-dependent endonuclease of influenza A and B viruses. It has shown nanomolar antiviral activity against influenza A and B viruses in vitro. In murine models of seasonal influenza and avian influenza A(H5N1) or A(H7N9), orally administered baloxavir showed a rapid reduction in pulmonary viral loads and decreased mortality. Baloxavir significantly reduced the time for alleviation of symptoms and reduced virus titres at 24 and 48 hours post-treatment at three different doses (10 mg, 20 mg and 40 mg) in a phase II study with patients experiencing uncomplicated influenza.
Mechanism of action
Baloxavir marboxil is an influenza therapeutic agent, specifically, an enzyme inhibitor targeting the influenza virus' cap-dependent endonuclease activity, one of the activities of the virus polymerase complex. In particular, it inhibits a process known as cap snatching, by which the virus derives short, capped primers from host cell RNA transcripts, which it then uses for polymerase-catalyzed synthesis of its needed viral mRNAs. A polymerase subunit binds to the host pre-mRNAs at their 5' caps, then the polymerase's endonuclease activity catalyzes its cleavage "after 10–13 nucleotides". As such, its mechanism is distinct from neuraminidase inhibitors such as oseltamivir and zanamivir.
Pharmacokinetics
Baloxavir marboxil is an antiviral drug that works against influenza virus to block viral replication. It has an 50% inhibitory concentration (IC50) of 1.4 to 3.1 nM for influenza A viruses and 4.5 to 8.9 nM for influenza B viruses in a polymerase acidic (PA) endonuclease assay. In murine models of influenza and avian influenza A, baloxavir reduced pulmonary viral loads and increased survival rates of mice. The reduction of viral titer was observed within 24 hours of administration, in a dose-dependent manner.
Side Effects
Common side effects following the single dose administration of baloxavir marboxil include diarrhea, bronchitis, common cold, headache, and nausea. Adverse events were reported in 21% of people who received baloxavir, 25% of those receiving placebo, and 25% of oseltamivir.
Metabolism
Baloxavir predominantly undergoes UGT1A3-mediated metabolism to form glucuronic acid conjugate. It is subsequently metabolized by CYP3A4 to form sulfoxide.
References
[1] HUGHES* D L. Review of the Patent Literature: Synthesis and Final Forms of Antiviral Drugs Tecovirimat and Baloxavir Marboxil[J]. Organic Process Research & Development, 2019, 23 7: 1298-1307. DOI:10.1021/acs.oprd.9b00144.
[2] ANDOYOSHINORI. Pharmacokinetic and pharmacodynamic analysis of baloxavir marboxil, a novel cap-dependent endonuclease inhibitor, in a murine model of influenza virus infection.[J]. Journal of Antimicrobial Chemotherapy, 2021, 76 1: 189-198. DOI:10.1093/jac/dkaa393.
[3] YANGTIANRUI. Baloxavir Marboxil: The First Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza.[J]. Annals of Pharmacotherapy, 2019, 53 7: 754-759. DOI:10.1177/1060028019826565.
[4] Chemical Structure and Synthesis of Baloxavir Marboxil, A Novel Endonuclease Inhibitor For The Treatment Influenza : An Overview
Properties of Baloxavir marboxil
| Boiling point: | 712.8±70.0 °C(Predicted) |
| Density | 1.57±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO:45.0(Max Conc. mg/mL);78.73(Max Conc. mM) |
| form | A crystalline solid |
| pka | -1.46±0.40(Predicted) |
| color | White to yellow |
Safety information for Baloxavir marboxil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Baloxavir marboxil
| InChIKey | HKVHAHZGMLTCDW-BWFGELNCSA-N |
| SMILES | C(O)(=O)OC(OC1=C2N(C=CC1=O)N([C@H]1C3=CC=C(F)C(F)=C3CSC3=CC=CC=C31)[C@]1([H])COCCN1C2=O)C |
Baloxavir marboxil manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

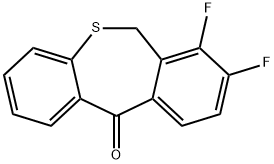
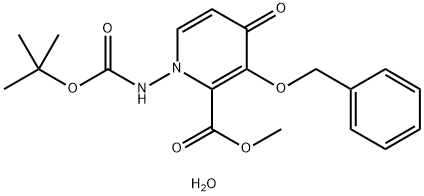
![(12aR)-7-(hexyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]Oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazine-6,8-dione](https://img.chemicalbook.in/CAS/20200611/GIF/2136287-67-5.gif)
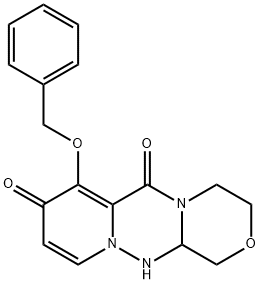
![(R)-7-(benzyloxy)- 3,4,12,12a-tetrahydro- 1H-[1,4]oxazino[3,4- c]pyrido[2,1-f][1,2,4]- triazine-6,8-dione](https://img.chemicalbook.in/CAS/20200611/GIF/1985607-70-2.gif)
You may like
-
 1985606-14-1 Baloxavir marboxil 99%View Details
1985606-14-1 Baloxavir marboxil 99%View Details
1985606-14-1 -
 1985606-14-1 98%View Details
1985606-14-1 98%View Details
1985606-14-1 -
 Baloxavir Marboxil In-House 98%View Details
Baloxavir Marboxil In-House 98%View Details
1985606-14-1 -
 Baloxavir marboxil 98%View Details
Baloxavir marboxil 98%View Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1