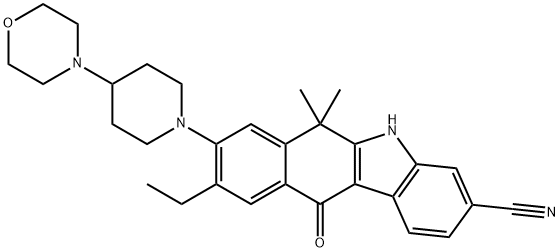
Alectinib
- CAS NO.:1256580-46-7
- Empirical Formula: C30H34N4O2
- Molecular Weight: 482.62
- MDL number: MFCD19440988
- EINECS: 821-541-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Alectinib?
Absorption
Alectinib reached maximal concentrations at 4 hours following administration of 600 mg twice daily under fed conditions in patients with ALK-positive NSCLC. The absolute bioavailability was 37% in the fed state. A high-fat, high-calorie meal increased the combined exposure of alectinib and its major metabolite M4 by 3.1-fold following oral administration of a single 600 mg dose.
Toxicity
The most common adverse reactions (>5%) associated with alectinib use were fatigue, constipation, edema, and myalgia. Less common effects associated with use were hepatotoxicity, interstitial lung disease (ILD)/pneumonitis, bradycardia, severe myalgia and creatine phosphokinase (CPK) elevation, and embryo-fetal toxicity. Females of reproductive potential are advised to use effective contraception during treatment with alectinib and for 1 week following the final dose.
Description
Alectinib is another selective ALK inhibitor that is able to overcome resistance mutations from prior crizotinib exposure [38].
Description
Alectinib, a drug developed in the past few years by a division of Hoffmann-La Roche, is an ALK inhibitor that was designed to treat a type of lung cancer. (ALK is the enzyme anaplastic lymphoma kinase.) A phase III study conducted this past February, however, was stopped early because the drug performed no better than its predecessor?crizotinib.
Amy W. Lasek at the University of Illinois, Chicago, and others discovered that ALK is also associated with alcohol dependence. She and her co-workers hypothesized that alectinib treatment might be a way to control the dependence.
When the researchers administered the drug to laboratory mice,?the animals drank less of a 20% ethanol solution than control mice. The control and test animals continued to drink nonalcoholic beverages in equal amounts, demonstrating that alectinib was specific for the alcohol pathway in the brain.
Chemical properties
White to Off-White Solid
The Uses of Alectinib
CH5424802 is a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth. CH5424802 have been cl inically evaluated for the treatment of patients with ALK-driven tumors.
The Uses of Alectinib
CH5424802 is a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth. CH5424802 have been clinically evaluated for the treatment of patients with ALK-driven tumors. Potent ALK inhibitor
Indications
Alectinib is a kinase inhibitor indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive, metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib. This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
What are the applications of Application
CH5424802 is an ATP-competitive inhibitor for ALK and L1196M
Background
Alectinib is a second generation oral drug that selectively inhibits the activity of anaplastic lymphoma kinase (ALK) tyrosine kinase. It is specifically used in the treatment of non-small cell lung cancer (NSCLC) expressing the ALK-EML4 (echinoderm microtubule-associated protein-like 4) fusion protein that causes proliferation of NSCLC cells. Inhibition of ALK prevents phosphorylation and subsequent downstream activation of STAT3 and AKT resulting in reduced tumour cell viability.
Approved under accelerated approval in 2015, alectinib is indicated for use in patients who have progressed on or were not tolerant of crizotinib, which is associated with the development of resistance.
Definition
ChEBI: An organic heterotetracyclic compound that is 6,6-dimethyl-5,6-dihydro-11H-benzo[b]carbazol-11-one carrying additional cyano, 4-(morpholin-4-yl)piperidin-1-yl and ethyl substituents at positions 3, 8 and 9 respectively. Used (as the hydr chloride salt) for the treatment of patients with anaplastic lymphoma kinase-positive, metastatic non-small cell lung cancer.
Indications
Alectinib (Alecensa(R), Roche) was approved first in Japan in 2014 and then by US FDA in 2015 as a second-generation ALK inhibitor for NSCLC treatment on patients who have progressed or do not tolerate crizotinib. Developed through a structure-based drug design approach, alectinib is a benzocarbazolone derivative that has shown potent inhibitory activity against ALK (IC50 value of 1.9 nM) and gatekeeper mutant L1196M ALK (IC50 value of 1.6 nM). Alectinib is effective with crizotinib-resistant ALK mutations on L1196M, F1174L, R1275Q, and C1156Y. In addition, an array of small-molecule inhibitors are currently being evaluated by several clinical trials for ALK-driven tumors.
brand name
Alecensa?
General Description
Class: receptor tyrosine kinase; Treatment: NSCLC; Oral bioavailability = 37%; Elimination half-life = 33 h; Protein binding = >99%
in vitro
in cell free assays the ic50 of ch5424802 for enzyme activity of alk was 1.9 nm; the dissociation constant (kd) value for alk in an atp-competitive manner was 2.4 nm. the inhibitory activity for two hot spot-activating mutations (f1174l and r1275q) in neuroblastoma was comparable to that for wildtype alk [1].
in vivo
in the nci-h2228 model, oral administration of ch5424802 resulted in dose-dependent tumor growth inhibition (ed50 = 0.46 mg/kg) and tumor regression. moreover, treatment of 20 mg/kg ch5424802 showed rapid tumor regression and tumor regrowth did not occur throughout the 4-week drug-free period [2].
Metabolism
Alectinib is metabolized by CYP3A4 to its major active metabolite M4. M4 is then further metabolized by CYP3A4. Both alectinib and M4 demonstrate similar in vivo and in vitro activity. In vitro studies suggest that alectinib is not a substrate for P-gp while M4 is.
References
1) Sakamoto?et al.?(2011),?CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant; Cancer Cell,?19?679 2) Kodama?et al.?(2014),?Alectinib Shows Potent Antitumor Activity against RET-Rearranged Non-Small Cell Lung Cancer., Mol. Cancer Ther.?13?2910 3) Lu?et al.?(2017),?The second-generation ALK inhibitor alectinib effectively induces apoptosis in human neuroblastoma cells and inhibits tumor growth in a TH-MYCN transgenic neuroblastoma mouse model; Cancer Lett,?400?61
Properties of Alectinib
| Melting point: | 274-276°C |
| Boiling point: | 722.5±60.0 °C(Predicted) |
| Density | 1.28 |
| storage temp. | -20° |
| solubility | Soluble in DMSO (up to 5 mg/ml with warming). |
| form | solid |
| pka | 13.70±0.40(Predicted) |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions are not stable and should be prepared fresh daily. |
Safety information for Alectinib
Computed Descriptors for Alectinib
Alectinib manufacturer
Aspen Biopharma Labs Pvt Ltd
Related products of tetrahydrofuran
![9-ethyl-6,6-diMethyl-8-(4-Morpholinopiperidin-1-yl)-11-oxo-5a,6,11,11a-tetrahydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride](https://img.chemicalbook.in/CAS/GIF/1256589-74-8.gif)
You may like
-
 1256580-46-7 Alectinib 98%View Details
1256580-46-7 Alectinib 98%View Details
1256580-46-7 -
 1256580-46-7 98%View Details
1256580-46-7 98%View Details
1256580-46-7 -
 1256580-46-7 98%View Details
1256580-46-7 98%View Details
1256580-46-7 -
 Alectinib >95% CAS 1256580-46-7View Details
Alectinib >95% CAS 1256580-46-7View Details
1256580-46-7 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3