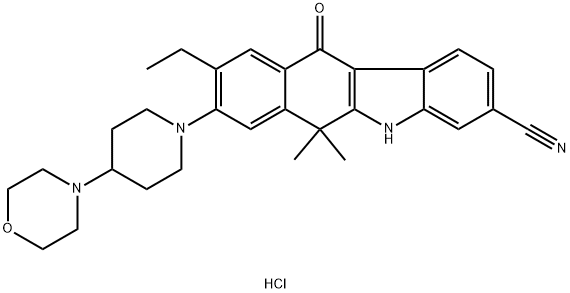
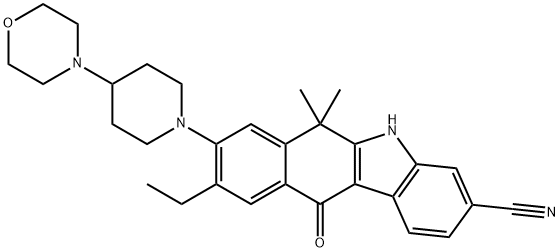
Alectinib Hydrochloride
- CAS NO.:1256589-74-8
- Empirical Formula: C30H35ClN4O2
- Molecular Weight: 519.09
- MDL number: MFCD27987893
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-07 19:25:08
What is Alectinib Hydrochloride?
Description
Alectinib hydrochloride, developed by Chugai Pharmaceutical/ Hoffman-La Roche under the trade name Alecensa®, was approved in Japan in April 2014 for the treatment of anaplastic lymphoma kinase (ALK) fusion-gene positive, unresectable, advanced, or recurrent non-small cell lung cancer (NSCLC). The compound is a highly selective second-generation ALK inhibitor, and while alectinib currently remains a focus of further development in Europe and the U.S., the compound has been granted orphan drug designation in Japan after showing a 93.5% objective response rate in phase II clinical trials. In addition to providing rapid treatment response time in a majority of patients, trials showed a 76% 2-year progression-free survival rate. Since the initial approval of crizotinib—the first ALK inhibitor indicated for treatment of ALKrearranged NSCLC —patients treated with crizotinib have shown remarkable improvement as compared to treatment with other chemotherapeutic methods,21 although drug resistance has shown to be a major side effect of this therapy. Preliminary preclinical and clinical studies of alectinib have shown significant promise for overcoming drug resistance developed with other ALK inhibitors.
The Uses of Alectinib Hydrochloride
CH5424802 Hydrochloride is a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth. Also is an intermediate of Alectinib (C183360), a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth.
Definition
ChEBI: Alectinib hydrochloride is a hydrochloride obtained by combining alectinib with one molar equivalent of hydrochloric acid. Used for the treatment of patients with anaplastic lymphoma kinase-positive, metastatic non-small cell lung cancer. It has a role as an antineoplastic agent and an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor. It contains an alectinib(1+).
Synthesis
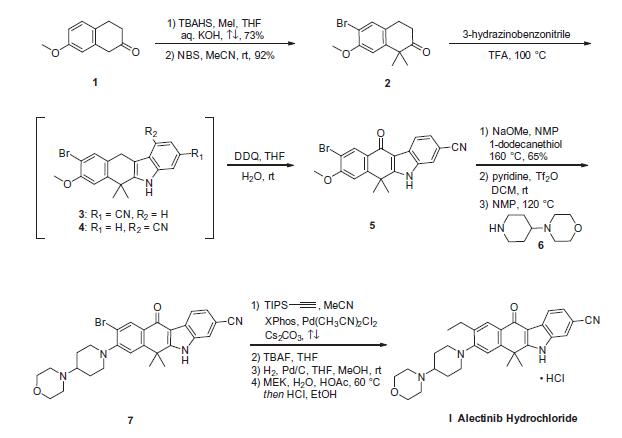
The synthetic route to alectinib as reported by Chugai begins with 7-methoxy-2-tetralone (1). Bis-methylation with tetrabutylammonium hydrogen sulfide (TBAHS)/aq KOH/MeI followed by bromination with N-bromosuccinimide (NBS) provided the bromo-tetralone 2 in 67% yield over the two steps. Further reaction of 2 with 3-hydrazinobenzonitrile/trifluoroacetic acid (TFA) led to formation of the desired Fischer indole product, albeit as a 1:1 mixture of regioisomers (3/4), which were carried forward as a mixture to oxidation with 2,3-dichloro-5,6-dicyano- 1,4-benzoquinone (DDQ). It is important to note that although representative procedures are published describing the conversion of 2 to alectinib (I), no yields were provided for these transformations. Following oxidation, the desired product 5 could be isolated as a single isomer via precipitation from the crude reaction mixture. Installation of the 4-morpholino-piperidine moiety took place in three transformations from 5, beginning with 1-dodecanethiol/ N-methyl-2-pyrrolidone (NMP)/NaOMe-facilitated methyl cleavage. The corresponding phenol was then readily converted to the triflate intermediate and displaced with 4-(piperidin-4-yl)morpholine (6) at elevated temperature, providing intermediate 7. Crosscoupling of the bromide 7 with ethynyl triisopropylsilane under Pd-catalyzed cross-coupling conditions (Pd(CH3CN)2Cl2/2-dicyclohexylphosphino- 20,40,60-triisopropylbiphenyl (XPhos), reflux) followed by cleavage of the resulting alkylsilane with tetrabutylammonium fluoride (TBAF) yielded the ethynyl precursor to alectinib. Hydrogenation of this unsaturated system under standard conditions (H2, Pd/C) followed by HCl salt formation furnished the final drug target alectinib hydrochloride (I).
Properties of Alectinib Hydrochloride
| storage temp. | Store at -20°C |
| solubility | DMSO:3.5(Max Conc. mg/mL);6.74(Max Conc. mM) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for Alectinib Hydrochloride
Computed Descriptors for Alectinib Hydrochloride
New Products
6-Bromo-3-iodo-1-methyl-1H-indazole 7-Bromo-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione N-octanoyl benzotriazole 4-Hydrazinobenzoic acid ELECTROLYTIC IRON POWDER 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 4-IODO BENZOIC ACID 4-Bromopyrazole 1-Boc-4-cyanopiperidine 5-BROMO-2CYANO PYRIDINE (R)-5-(2-aminopropyl)-2-methoxybenzenesulfonamide 5,6-Dimethoxyindanone Cycloleucine 1-Aminocyclobutanecarboxylic acid 1-Amino-1-cyclohexanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride TETRABUTYLAMMONIUM CYANIDE 4-bromo-3,5-dimethylbenzenesulfonyl chloride N-(5-Amino-2-methylphenyl)acetamide 4-EthylbenzylamineRelated products of tetrahydrofuran



You may like
-
 Alectinib Hydrochloride >95% CAS 1256589-74-8View Details
Alectinib Hydrochloride >95% CAS 1256589-74-8View Details
1256589-74-8 -
 4-N Butyl Resorcinol 18979-61-8 NLT 98%View Details
4-N Butyl Resorcinol 18979-61-8 NLT 98%View Details
18979-61-8 -
 719-22-2 2,6- Di-Tert-Butyl-p-Benzoquinone NLT 98%View Details
719-22-2 2,6- Di-Tert-Butyl-p-Benzoquinone NLT 98%View Details
719-22-2 -
 1,3 Cyclohexane dione NLT 98%View Details
1,3 Cyclohexane dione NLT 98%View Details
504-02-9 -
 52-52-8 Cycloleucine 98+View Details
52-52-8 Cycloleucine 98+View Details
52-52-8 -
 2756-85-6 98+View Details
2756-85-6 98+View Details
2756-85-6 -
 1-Aminocyclobutanecarboxylic acid 98+View Details
1-Aminocyclobutanecarboxylic acid 98+View Details
22264-50-2 -
![1841081-72-8 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride 98+](https://img.chemicalbook.in//Content/image/CP5.jpg) 1841081-72-8 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride 98+View Details
1841081-72-8 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride 98+View Details
1841081-72-8