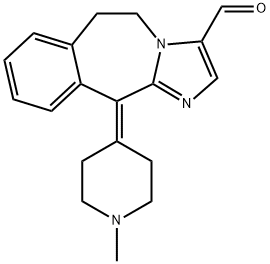
ALCAFTADINE
- CAS NO.:147084-10-4
- Empirical Formula: C19H21N3O
- Molecular Weight: 307.39
- MDL number: MFCD09954106
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is ALCAFTADINE?
Description
Alcaftadine, a histamine H1/H2 receptor antagonist, was approved in the United States in 2010 for the prevention of itching and redness associated with allergic conjunctivitis. Seasonal and perennial allergic conjunctivitis affects up to 40% of the population worldwide. There are numerous treatment options, with topical antihistamines being an effective therapy. Some of the primary symptoms and signs of allergic conjunctivitis are ocular itching and conjunctival redness. The pharmaceutical market for conjunctivitis is substantial and steadily increasing.
Originator
Janssen Research Foundation (United States)
The Uses of ALCAFTADINE
Alcaftadine is a H1 histamine receptor antagonist. Alcaftadine is used to prevent eye irritation and treat the signs and symptoms of allergic conjunctivitis.
Background
Alcaftadine is a H1 histamine receptor antagonist indicated for the prevention of itching associated with allergic conjunctivitis. This drug was approved in July 2010.
Indications
For the prevention of itching associated with allergic conjunctivitis.
Definition
ChEBI: An imidazobenzazepine that is 6,11-dihydro-5H-imidazo[2,1-b][3]benzazepine substituted at position 3 by a formyl group and at position 11 by a 1-methylpiperidin-4-ylidene group. An antihistamine used for treatment of allergi conjunctivitis.
brand name
LastacaftTM
Pharmacokinetics
Following bilateral topical ocular administration of alcaftadine ophthalmic solution, 0.25%, the mean plasma Cmax of alcaftadine was approximately 60 pg/mL and the median Tmax occurred at 15 minutes. Plasma concentrations of alcaftadine were below the lower limit of quantification (10 pg/mL) by 3 hours after dosing. The mean Cmax of the active carboxylic acid metabolite was approximately 3 ng/mL and occurred at 1 hour after dosing. Plasma concentrations of the carboxylic acid metabolite were below the lower limit of quantification (100 pg/mL) by 12 hours after dosing.
Clinical Use
Alcaftadine, an ophthalmic histamine H1 receptor antagonist, was approved by the FDA for the prevention of itching associated with allergic conjunctivitis and was launched under the trade name Lastacaft in early 2011. Alcaftadine was discovered by Janssen Pharmaceuticals and marketed by Vistakon Pharmaceuticals, both subsidiaries of Johnson & Johnson. However, unlike other marketed drugs, the synthesis of alcaftadine was only mentioned in the patents filed by Janssen’s scientists approximately twenty years ago.
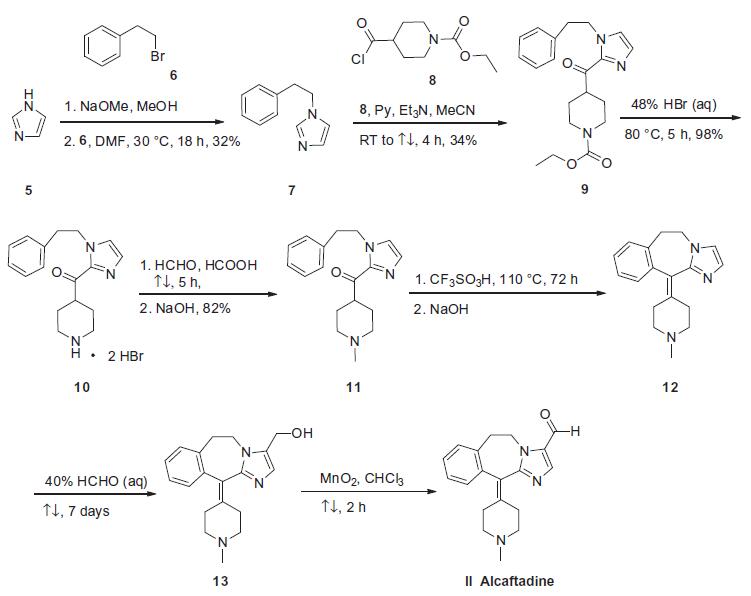
Synthesis
The synthetic route described in the scheme is based on the discovery route disclosed in those patents. 1-(2-Phenylethyl)- 1H-imidazole 7 is now commercially available, otherwise it could be prepared by reacting imidazole (5) with 2-phenylethyl bromide (6). With pyridine and triethylamine as base, imidazole 7 was reacted with acyl chloride 8 to provide piperidinecarboxylate 9 in 34% yield, followed by acid hydrolysis with 48% HBr aqueous solution to obtain piperidine dihydrobromide 10 in 98% yield. The N-methylation of 10 was acheived by Leuckart reaction with formaldehyde and formic acid to give 4-methylpiperidine 11 in 82% yield. Treatment of 11 with trifluoromethanesulfonic acid followed by subsequent basification triggered an intramolecular alkylation¨Cdehydration reaction to generate benzazepine 12. Next, alcohol 13 was obtained by prolonged exposure (7 days) of 12 to hydroxymethylation conditions using 40% aqueous formaldehyde. Oxidation of 13 with manganese (IV) oxide provided alcaftadine (II). The yields of last three steps from compound 11 to alcaftadine (II) were not provided in the patent.
Metabolism
The metabolism of alcaftadine is mediated by non-CYP450 cytosolic enzymes to the active carboxylic acid metabolite.
Properties of ALCAFTADINE
| Melting point: | 167 °C |
| Boiling point: | 556.2±60.0 °C(Predicted) |
| Density | 1.24 |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; Ethanol: 30 mg/ml; Ethanol:PBS(pH 7.2) (1:1): 0.5 mg/ml |
| form | powder to crystal |
| pka | 8.76±0.20(Predicted) |
| color | White to Yellow to Orange |
Safety information for ALCAFTADINE
Computed Descriptors for ALCAFTADINE
ALCAFTADINE manufacturer
Ralington Pharma
KPS Chemicals And Pharmaceuticals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![11-(1-Methylpiperidin-4-ylidene)-6,11-dihydro-5H-benzo[d]iMidazo[1,2-a]azepine](https://img.chemicalbook.in/CAS/20150408/GIF/147083-36-1.gif)
You may like
-
 Alcaftadine 98%View Details
Alcaftadine 98%View Details
147084-10-4 -
 Alcaftadine 147084-10-4 99%View Details
Alcaftadine 147084-10-4 99%View Details
147084-10-4 -
 Alcaftadine CAS 147084-10-4View Details
Alcaftadine CAS 147084-10-4View Details
147084-10-4 -
 147084-10-4 99%View Details
147084-10-4 99%View Details
147084-10-4 -
 147084-10-4 Alcaftadine 98%View Details
147084-10-4 Alcaftadine 98%View Details
147084-10-4 -
 Alcaftadine 98%View Details
Alcaftadine 98%View Details
147084-10-4 -
 Alcaftadine 98% (HPLC) CAS 147084-10-4View Details
Alcaftadine 98% (HPLC) CAS 147084-10-4View Details
147084-10-4 -
 Alcaftadine CAS 147084-10-4View Details
Alcaftadine CAS 147084-10-4View Details
147084-10-4