Nitazoxanide
Synonym(s):Nitazoxanide;NTZ; 2-(Acetyloxy)-N-(5-nitro-2-thiazolyl)benzamide
- CAS NO.:55981-09-4
- Empirical Formula: C12H9N3O5S
- Molecular Weight: 307.28
- MDL number: MFCD00416599
- EINECS: 259-931-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-27 17:19:27
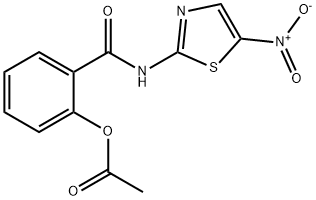
What is Nitazoxanide?
Description
Nitazoxanide (NT Z) has been approved as an orphan drug for the treatment of diarrhea in children (age, 1–11 years) and is associated with giardiasis, but it also is approved for diarrhea caused by crytosporidiosis in patients with AIDS. Crytosporidiosis is a protozoal infection caused by Cryptosporidi um parvum. The condition is uncommon in healthy individuals but can be life-threatening in immunosuppressed patients and those with HIV infections.
Chemical properties
Crystalline Solid
Originator
Alinia,Romark Laboratories, L.C.
The Uses of Nitazoxanide
Nitazoxanide has been used:
- to test its anti-viral activity against chikungunya virus
- as an antiprotozoal agent to test its effect on cell viability in various cancer cell lines
- to test its effect on human cytomegalovirus (HCMV) infected human fibroblast HFF cells
The Uses of Nitazoxanide
An anthelmintic (cestodes), antiprotozoal (cryptosporidium). Kills Mycobacterium tuberculosis.
The Uses of Nitazoxanide
antihypertensive
The Uses of Nitazoxanide
For the treatment of diarrhea in adults and children caused by the protozoa Giardia lamblia and for the treatment of diarrhea in children caused by the protozoa Cryptosporidium parvum.
Background
Nitazoxanide belongs to the class of drugs known as thiazolides. Nitazoxanide (NTZ) is a broad-spectrum anti-infective drug that markedly modulates the survival, growth, and proliferation of a range of extracellular and intracellular protozoa, helminths, anaerobic and microaerophilic bacteria, in addition to viruses. This drug is effective in the treatment of gastrointestinal infections including Cryptosporidium parvum or Giardia lamblia in healthy subjects. It is generally well tolerated. Nitazoxanide is a first-line, standard treatment for illness caused by C. parvum or G. lamblia infection in healthy (not immunosuppressed) adults and children and may also be considered in the treatment of illnesses caused by other protozoa or helminths . Recently, this drug has been studied as a broad-spectrum antiviral agent due to its ability to inhibit the replication of several RNA and DNA viruses .
Indications
For the treatment of diarrhea in adults and children caused by the protozoa Giardia lamblia, and for the treatment of diarrhea in children caused by the protozoan, Cryptosporidium parvum .
Nitazoxanide has not been shown to be superior to placebo medication for the management of diarrhea caused by Cryptosporidium parvum in patients with HIV/immunodeficiency .
Definition
ChEBI: Acetic acid [2-[[(5-nitro-2-thiazolyl)amino]-oxomethyl]phenyl] ester is a carboxylic ester and a member of benzamides. It is functionally related to a salicylamide.
Manufacturing Process
To a solution containing one mole p-metoxy-benzoyl chloride and one mole of carefully purified 2-amino-5-nitro-triazole in 200 ml of anhydrous tetrahydrofuran, one mole of triethylamine has been slowly added (about 10 minutes) while stirring. The reaction mixture, which became slightly warm, was stirred during 45 minutes and then poured under agitation, into 2 liters of distilled water. The stirring was continued until the precipitation of salicylamide, N-(5-nitro-2-thiazolyl)-, acetate (ester) was complete. The obtained precipitate was dried, washed with water, dried again and recrystallized from methanol. The yield about 60%; melting point 202°C.
brand name
Alinia (Romark).
Therapeutic Function
Anthelmintic
Antimicrobial activity
In vitro Cryptosporidium parvum sporocytes and oocysts are inhibited by <33 μm, and Giardia lamblia (intestinalis) trophozoites by <10 μm. The metabolite tizoxanide is more active than the parent compound against some isolates. E. histolytica is inhibited by 6–23 μm (parent compound) and 5.6– 28 μm (metabolite), and T. vaginalis by 0.5–15.5 μm (parent compound) and 0.3–12.2 μm (metabolite). Activity against other micro- organisms, including some helminths, bacteria (Clostridium difficile) and viruses (hepatitis C) has also been demonstrated.
Acquired resistance
Resistance caused by altered expression of genes involved in stress response has been demonstrated in experimental studies with G. lamblia.
General Description
Nitazoxanide (NTZ), a thiazolide compound is a antiparasitic drug with structure similar to niclosamide.
Pharmaceutical Applications
A synthetic broad-spectrum antiparasitic nitroheterocycle (2-acetyloxy- N-(5-nitro-2-thiazolyl) benzamide), formulated for oral use.
Biochem/physiol Actions
Nitazoxanide is an inhibitor of pyruvate-ferredoxin oxidoreductase (PFOR); Antimicrobial recently found to kill both non-replicating and replicating mycobacteria. FDA approved anti-parasitic drug (2002). Recent work (C & EN Sept. 14, 2009, p. 28) highlights that NTZ kills non-replicating and replicating TB bacteria and no apparent resistance is detected.
Mechanism of action
Nitazoxanide is a pro-drug that is metabolically converted into the deactylated drug tizoxanide (TIZ). The TIZ then undergoes a four-electron reduction of the 5-nitro group giving various short-lived intermediates, which may include the hydroxylamine derivative. It is these reduced products that represent the active form of NTZ. Whereas these intermediates would suggest that NTZ has the same mechanism of action as metronidazole, this does not appear to be the case. Nitazoxanide is thought to inhibit the enzyme pyruvate:ferredoxin oxidoreductase in Trichomonas vaginali s, Entamoeba histolytica, and Cl ostridium perfingens. The results of this inhibition is disruption of the bioenergetics of these organisms. Unlike metronidazole and tinidazole, which fragment DNA and are suspected mutagenic agents, NTZ and TIZ do not cause DNA fragmentation and are not considered to be mutagenic. This might be associated with the higher redox potential found for NTZ, a nitrothiazole, in comparison with very low redox potential found for the nitroimidazoles, such as metronidazole and tinidazole. Additional metabolites of TIZ also includes the glucuronide, which shows some biological activity, and small amounts of an aromatic hydroxylation product.
Pharmacokinetics
The general effect of this medication is the prevention of microbe activity through disruption of important energy pathways for survival and proliferation . Nitazoxanide exhibits antiprotozoal activity by interfering with the pyruvate ferredoxin/flavodoxin oxidoreductase dependent electron transfer reaction, an essential reaction need for anaerobic energy metabolism of various microorganisms. Sporozoites of Cryptosporidium parvum and trophozoites of Giardia lamblia are therefore inhibited, relieving symptoms of diahrrea . Interference with the PFOR enzyme-dependent electron transfer reaction may only be one of the many pathways by which nitazoxanide exhibits antiprotozoal activity .
Pharmacokinetics
After oral administration the major circulating metabolites are tizoxanide (desacetyl nitazoxanide) and its glucuronide. Minor metabolites include salicyluric acid and tizoxanide sulfate. Maximum concentrations of the active metabolites tizoxanide and tizoxanide glucuronide are observed within 1–4 h. Following a single oral dose of 500 mg given with food, the Cmax of both metabolites was around 10 mg/L. Tizoxanide has a halflife of around 1–2 h and is >99.9% bound to plasma proteins.
Clinical Use
It is indicated for the treatment of diarrhea caused by G. lamblia or C. parvum.
Side Effects
Nitazoxanide appears well tolerated. Side effects may include abdominal pain diarrhea, headache and nausea.
Veterinary Drugs and Treatments
Nitazoxanide oral paste is indicated for the treatment of horses with
equine protozoal myeloencephalitis (EPM) caused by Sarcocystis
neurona.
In humans, nitazoxanide is approved (in the USA) for use in
treating diarrhea caused by Cryptosporidium parvum and Giardia
lamblia in pediatric patients from ages 1 to 11 years old.
Because of the drug’s spectrum of activity and apparent safety,
there is considerable interest in using it in a variety of companion
animal species, but data is lacking for specific indications and
dosages.
Absorption
The relative bioavailability of the suspension compared to the tablet was 70%. When administered with food the AUC and Cmax increased by two-fold and 50%, respectively, for the tablet and 45 to 50% and ≤ 10%, respectively, for the oral suspension .
Metabolism
The active metabolite of this drug is tizoxanide (desacetyl-nitazoxanide). The initial reaction in the metabolic pathway of Nitazoxanide is hydrolysis to tizoxanide, followed by conjugation, primarily by glucuronidation to tizoxanide glucuronide.
The oral suspension bioavailability of this drug is not equivalent to that of the oral tablets. Compared to the to the tablet, the bioavailability of the suspension was 70% .
When administered with food, the AUCt of tizoxanide and tizoxanide glucuronide in plasma is increased to almost two-fold and the maximum concentration is increased by almost 50% compared to when ingested without food .
When the oral suspension was ingested with food, the AUC of tizoxanide and tizoxanide glucuronide increased by approximately 50% and the Cmax increased by less than 10% .
Metabolism
Nitazoxanide is available as powder that is reconstituted and dispensed as an oral suspension. The drug is well absorbed from the GI tract and rapidly metabolized, with elimination products appearing in the urine and feces. The only identified products in the plasma are TIZ and its glucuronide. The product can be taken with food.
Side Effects
The side effects of nitazoxanide do not significantly differ from a placebo treatment for giardiasis;these symptoms include stomach pain, headache, upset stomach, vomiting, discolored urine, excessive urinating, skin rash, itching, fever, flu syndrome, and others. Nitazoxanide does not appear to cause any significant adverse effects when taken by healthy adults.
Toxicity
Data on nitazoxanide overdosage is not available . In studies in rodents and dogs, the oral LD50 was higher than 10,000 mg/kg. One-time oral doses of up to 4000 mg nitazoxanide have been given to healthy adult volunteers without severe adverse effects. Gastric lavage may be appropriate soon after oral administration if overdose occurs. Supportive and symptomatic treatment should also be administered .
According to previous studies , less than 1% of the patients age 12 years and older participating in clinical trials with NTZ suffered from the following adverse effects:
Systemic: asthenia, fever, pain, allergic reaction, pelvic pain, back pain, chills, fever, flu-like syndrome.
Central Nervous System: dizziness, somnolence, insomnia, tremor, hypesthesia.
Gastrointestinal System: vomiting, dyspepsia, anorexia, flatulence, constipation, dry mouth, thirst.
Urogenital System: discolored urine, dysuria, amenorrhea, metrorrhagia, kidney pain, edema labia.
Metabolic & Nutrition: increased SGPT.
Hemic & Lymphatic Systems: anemia, leukocytosis.
Skin: rash, pruritus.
Special Senses: eye discoloration, ear ache.
Respiratory System: epistaxis, lung disease, pharyngitis.
Cardiovascular System: tachycardia, syncope, hypertension.
Muscular System: myalgia, leg cramps, spontaneous bone fracture.
storage
Store at -20°C
References
References/Citations:
Properties of Nitazoxanide
| Melting point: | 202°C |
| Density | 1.629 g/cm3 |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (>50 mg/ml) |
| form | solid |
| pka | 6.18±0.50(Predicted) |
| color | Off-white |
| λmax | 416nm(Phosphate buffer sol.)(lit.) |
| Merck | 14,6567 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 55981-09-4(CAS DataBase Reference) |
Safety information for Nitazoxanide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
Computed Descriptors for Nitazoxanide
Nitazoxanide manufacturer
Heer Pharma Pvt Ltd
Related products of tetrahydrofuran
You may like
-
 Nitazoxanide 99%View Details
Nitazoxanide 99%View Details -
 NITAZOXANIDE 99%View Details
NITAZOXANIDE 99%View Details -
 Nitazoxanide 98% CAS 55981-09-4View Details
Nitazoxanide 98% CAS 55981-09-4View Details
55981-09-4 -
 Nitazoxanide 55981-09-4 98%View Details
Nitazoxanide 55981-09-4 98%View Details
55981-09-4 -
 55981-09-4 95-99%View Details
55981-09-4 95-99%View Details
55981-09-4 -
 Nitazoxanide CAS 55981-09-4View Details
Nitazoxanide CAS 55981-09-4View Details
55981-09-4 -
 Nitazoxanide CAS 55981-09-4View Details
Nitazoxanide CAS 55981-09-4View Details
55981-09-4 -
 Nitazoxanide CAS 55981-09-4View Details
Nitazoxanide CAS 55981-09-4View Details
55981-09-4