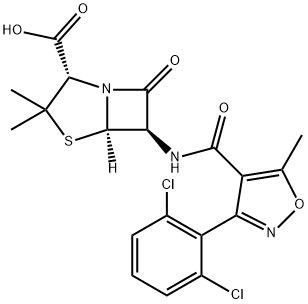
Aspoxicillin
- CAS NO.:63358-49-6
- Empirical Formula: C21H27N5O7S
- Molecular Weight: 493.53
- MDL number: MFCD01724985
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
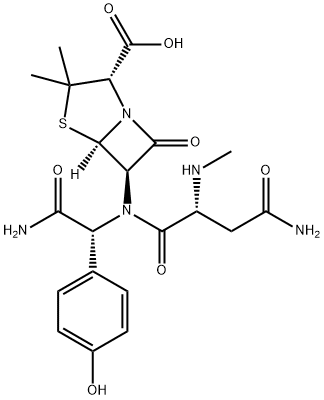
What is Aspoxicillin?
Description
Aspoxicillin is an injectable, amino acid-type penicillin highly active against Gram-positive and Gram-negative bacteria, including the β-lactamase producing Bacillus frugilis. It is reportedly effective in the treatment of peritonitis, pneumonia and bronchitis.
Originator
Tanabe (Japan)
The Uses of Aspoxicillin
Aspoxicillin is a broad-spectrum antibiotic.
Definition
ChEBI: Aspoxicillin is a peptide.
Manufacturing Process
1). 3 g (23.6 millimoles) of D-2-amino-3-N-methylcarbamoyl-propionic acid
hydrochloride (D-N'-methylasparagine HCl), 4.5 g of benzyloxycarbonyl
chloride, 30 g of water, 30 ml of tetrahydrofuran and 12 g of potassium
carbonate are mixed at 5° to 10°C. Then, the mixture is stirred at the same
temperature for 2 hours. During the reaction, the mixture is kept at a slightly
alkaline pH (pH 8) with potassium carbonate. 10 ml of water are added to the
reaction mixture, and insoluble materials are filtered off. The filtrate is washed
twice with ethyl acetate, acidified with citric acid and then extracted three
times with 50 ml of ethyl acetate. The ethyl acetate extracts are washed with
water, dried and evaporated to remove solvent. 5.1 g of D-2-
benzyloxycarbonylamino-3-N-methylcarbamoyl-propionic acid are obtained.
MP: 142°-143°C.
2). 2.8 g (10 millimoles) of D-2-benzyloxycarbonylamino-3-Nmethylcarbamoyl-
propionic acid, 2.27 g of dicyclohexylcarbodiimide, 1.27 g of
N-hydroxysuccinimide and 120 ml of tetrahydrofuran are mixed at 0° to 5°C,
and the mixture is stirred at the same temperature for 16 hours. Insoluble
materials are filtered off. Then, the filtrate is evaporated at 20°C under
reduced pressure to remove the solvent, and the crystalline precipitates thus
obtained are washed with a mixture of benzene-ether. 2.6 g of N-(D-2-
benzyloxycarbonylamino-3-N-methylcarbamoyl-propionyloxy)succinimide are
obtained. MP: 132°-134°C.
3). 3.75 g of N-(D-2-benzyloxycarbonylamino-3-Nmethylcarbamoylpropionyloxy)
succinimide are dissolved in 50 ml of
tetrahydrofuran. 12.5 ml of an aqueous 1 N-sodium hydroxide solution
containing 2.3 g of p-hydroxy-D-phenylglycine are added to the solution. The
mixture is stirred at room temperature for 24 hours. 20 ml of ethyl acetate
and 20 ml of water are added to the reaction mixture, and said mixture is
shaken. Then, the aqueous layer is separated, adjusted to pH 3 with citric acid and extracted with a mixture of 20 ml of tetrahydrofuran and 10 ml of ethyl
acetate. The extract is washed with water, dried and evaporated to remove
solvent. The residue thus obtained is washed with ether. 3.0 g of D-2-(D-2-
benzyloxycarbonylamino-3-N-methylcarbamoylpropionamido)- 2-phydroxyphenylacetic
acid are obtained as colorless crystalline powder. MP:
154°-156°C(decomp.).
4). 429 mg of D-2-(D-2-benzyloxycarbonylamino-3-Nmethylcarbamoylpropionamido)-
2-p-hydroxyphenylacetic acid and 382 mg of
6-aminopenicillanic acid triethylamine salt are dissolved in 10 ml of
dimethylformamide. 303 mg of diphenylphosphoric azide [N3PO(OC6H5)2] and
110 mg of triethylamine are added to the solution at -5°C, and the mixture is
stirred at -5°C for 15 hours. After the reaction, the mixture is adjusted to pH
3 with an aqueous 5% citric acid solution and extracted with a mixture of 15
ml of tetrahydrofuran and 10 ml of ethyl acetate. The extract is washed with
water, dried and then evaporated at below 40°C to remove the solvent. Ether
is added to the residue obtained, and precipitates are collected by filtration.
509 mg of 6-[D-2-(D-2-benzyloxycarbonylamino-3-Nmethylcarbamoylpropionamido)-
2-p-hydroxyphenylacetamido]penicillanic acid
are obtained as a colorless powder.
5). 627 mg of 6-[D-2-(D-2-benzyloxycarbonylamino-3-N-methylcarbamoylpropionamido)-
2-p-hydroxyphenylacetamido]penicillanic acid and 400 mg of
30% palladium-BaCO3 are suspended in 10 ml of methanol. The suspension is
shaken at room temperature for 30 minutes. Said shaking step is carried out
in a hydrogen gas atmosphere under atmospheric pressure. After the reaction
is completed, the catalysts are removed by filtration. The filtrate is evaporated
at below 40°C to remove the solvent, and ether is added to the residue. Then,
a colorless crystalline powder is collected by filtration and washed with
tetrahydrofuran. 443 mg of 6-[D-2-(D-2-amino-3-N-methylcarbamoylpropionamido)-
2-p-hydroxyphenylacetamido]penicillanic acid are obtained.
MP: 198°-201°C(decomp.).
brand name
Doyle
Therapeutic Function
Antibiotic
Antimicrobial activity
An acylaminopenicillin, synthesized from amoxicillin. It is
more active than carbenicillin against Ps. aeruginosa and is less
active than piperacillin against Staph. aureus, H. influenzae,
the Enterobacteriaceae and Ps. aeruginosa. It is not absorbed
when dosed orally; the plasma half-life is 87 min after intravenous
infusion.
Aspoxicillin has been used in the treatment of respiratory,
skin and soft tissue and urinary infections in adults and
children, and, in combination with aminoglycosides, against
gynecological infections and infections in patients with hematological
disorders.
Properties of Aspoxicillin
| Melting point: | 195-198° (dec) |
| Boiling point: | 985.1±65.0 °C(Predicted) |
| Density | 1.51±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly, Sonicated), Methanol (Slightly) |
| form | neat |
| pka | 2.44±0.50(Predicted) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Water : 25 mg/mL (50.66 mM; Need ultrasonic) |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 63358-49-6 |
Safety information for Aspoxicillin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Aspoxicillin
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4