Coenzyme B12
Synonym(s):5′-Deoxyadenosylcobalamine;Adenosyl cobalamine;Cobamamide;DMBC coenzyme
- CAS NO.:13870-90-1
- Empirical Formula: C72H99CoN18O17P
- Molecular Weight: 1578.6
- MDL number: MFCD00135609
- EINECS: 237-627-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-06 22:41:32
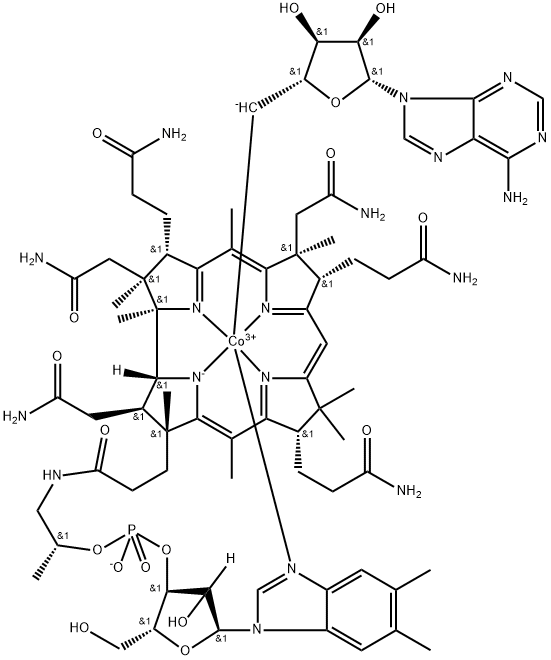
What is Coenzyme B12?
Description
Coenzyme B12 (adenosylcobalamin; AdoCbl; 5'-Deoxyadenosylcobalamin) is a form of vitamin B12. It belongs to the corrinoid group of compounds containing a corrin macrocycle and are produced only by certain bacteria and archaea. It is a cofactor for various enzymes, including mutases, eliminases, aminomutases, and reductases. These enzymes catalyze reactions that generate free radicals through the release of the adenosyl group, allowing usually unreactive molecules to become reactive. Genetic mutations in enzymes synthesizing Coenzyme B12 lead to Coenzyme B12 deficiency and methylmalonic aciduria.
Originator
Actimide,Tobishi
The Uses of Coenzyme B12
emulsifying agent
The Uses of Coenzyme B12
Coenzyme B12 has been used as a supplement for culturing plasmid variants in in vivo assay of yvrC-lacZ fusions.
Definition
ChEBI: A member of the class of cobalamins that is vitamin B12 in which the cyano group is replaced by a 5'-deoxyadenos-5'-yl moiety. It is one of the two metabolically active form of vitamin B12.
Definition
Coenzyme B12 (5'-Deoxy-5'-adenosylcobalamin or Ado-Cbl) is the largest molecule among the B type vitamins. It is an essential cofactor in many biological rearrangement reactions. Coenzyme B12 activates enzymes such as methionine synthase (MetH), methyl-malonyl-CoA mutase (MutAB), and glycerol dehydratase (DhaB). Biologically, coenzyme B12 is one of the two main types of vitamin B12, adenosyl-cobalamin and methyl-cobalamin, and the former is highly preferred for many enzymes, including glycerol and diol dehydratases[1].
Manufacturing Process
Isopropylidine adenosine was converted to the p-toluene sulphonyl (tosyl)
ester by reaction with tosyl chlorine solution, following the method of Clark et
al. (1951) [J. Chem. Soc. 2952]. Because of its tendency to cyclization, the
reagent was used directly it was ready. A reaction flask with separating
funnels was set up in such a way that the whole system could be evacuated
and filled with pure nitrogen two or three times, to eliminate all oxygen, and
reagents could then be added when desired, in the closed system.
The flask contained 700.0 mg hydroxocobalamin in 20 ml of water, one funnel
200.0 mg sodium borohydride in 10 ml of water, and another the crude
isopropylidine adenosine tosyl ester made from 500 mg isopropylidine
adenosine dissolved in 10 ml of 50% aqueous methanol. On adding the
borohydride to the vitamin, the color changed instantly from red to brown,
then slowly to a greenish black. After 15 min the isopropylidine adenosine
tosyl ester was added, and the colour slowly changed to a red-brown. After 45
min at room temperature air was admitted and the mixture was shaken to
reoxidise any remaining reduced vitamin B12. The alkaline solution was
neutralized with dilute hydrochloric acid and extracted with phenol carbon
tetrachloride 3:1 in small portions till the aqueous layer was nearly colorless.
The combined extracts were washed with water, mixed with about ten parts of
carbon tetrachloride-acetone 10:1 and shaken with small portions of water till
all red color was removed.
The product was purified by chromatography on columns of DEAE (diethyl
aminoethyl) cellulose (3 x 1) followed by CM (carboxymethyl) cellulose (6 x
1), developed with water. Nearly all the color washed quickly through DEAE
cellulose. The effluent and washes were applied to the CM cellulose column,
which was further developed with water. Elution was continued as long as this
fraction continued to emerge, in a total of 850 ml. One half of this fraction
(425 ml) was concentrated to a few ml under reduced pressure; it crystallized
slowly after adding acetone to slight turbidity. So cobamamide was obtained.
Therapeutic Function
Anabolic, Analgesic
Biotechnological Production
There exist two distinctive routes for the biosynthesis of coenzyme B12, namely oxygen-dependent (encoded by cob operons) and oxygen-independent (by CBI operons) pathways. Their requirement of molecular oxygen during synthesis and the point at which cobalt is inserted into the corrin ring. De novo coenzyme B12 biosynthesis is limited to some bacteria and archaea. The aerobic pathway is present in Pseudomonas denitrificans, Sinorhizobium meliloti, Rhodobacter sphaeroides and Pseudomonas aeruginosa. At the same time, the anaerobic one is present in Salmonella typhimurium, Klebsiella pneumoniae, Citrobacter amalonaticus, Bacillus megaterium, Propionibacterium shermanii and Lactobacillus reuteri.
General Description
Vitamin B12 cobalamin refers to a group of chemically-related cobalt containing molecules. The physiologically active forms of vitamin B12 include methylcobalamin and adenosylcobalamin, whereas hydroxocobalamin (vitamin B12a, OHCbl) and cyanocobalamin (CNCbl) are storage and delivery forms.
Agricultural Uses
Cobamide enzyme is the cobalt complex formed between the cobalt-porphyrin ring structure and the nucleotide in vitamin B12 co-enzyme.
Biochem/physiol Actions
Vitamin B12 cobalamin is involved in DNA synthesis and fatty acid synthesis. It also plays a vital role as a coenzyme in the conversion of mitochondrial methylmalonyl co-enzyme A to succinyl co-enzyme A.
References
[1] Thuan Phu Nguyen-Vo. “Analysis and characterization of coenzyme B12 biosynthetic gene clusters and improvement of B12 biosynthesis in Pseudomonas denitrificans ATCC 13867.” Fems Microbiology Letters 365 21 (2018).
[2] I. I. Merkelbach, Hm Henk Buck. “Mechanism of action of coenzyme B12. Quantum-chemical considerations.” Recueil des Travaux Chimiques des Pays-Bas 28 1 (2010): 166–169.
[3] Prof. Dr. Karl Gruber, Prof. Dr. Bernhard Krutler. “Coenzyme B12 Repurposed for Photoregulation of Gene Expression.” Angewandte Chemie International Edition 55 19 (2016): 5638–5640.
Properties of Coenzyme B12
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly, Sonicated) |
| pka | 3.5(at 25℃) |
| form | Solid |
| color | Red to Very Dark Red |
| Water Solubility | 26g/L(24 ºC) |
| Merck | 13,2476 |
| BRN | 4122932 |
| Stability: | Hygroscopic |
Safety information for Coenzyme B12
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Coenzyme B12
| InChIKey | WAJLPPDUWGAMTH-NGQUPMDYNA-L |
Coenzyme B12 manufacturer
Deccan Nutraceuticals Pvt. Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
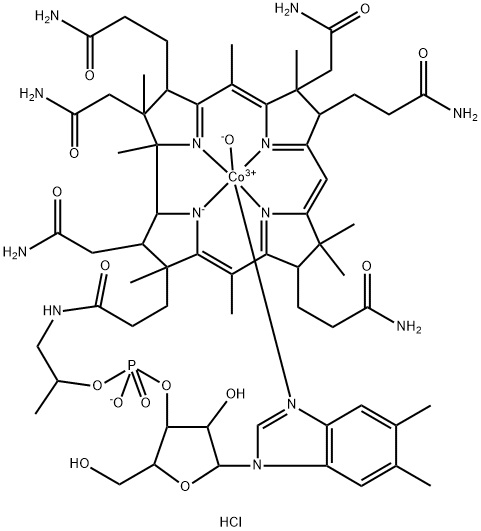
You may like
-
 13870-90-1 Cobamamide 98%View Details
13870-90-1 Cobamamide 98%View Details
13870-90-1 -
 13870-90-1 98%View Details
13870-90-1 98%View Details
13870-90-1 -
 Coenzyme B12 97% (HPLC) CAS 13870-90-1View Details
Coenzyme B12 97% (HPLC) CAS 13870-90-1View Details
13870-90-1 -
 Coenzyme B12 CAS 13870-90-1View Details
Coenzyme B12 CAS 13870-90-1View Details
13870-90-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1