3-O-Ethyl-L-ascorbic acid
- CAS NO.:86404-04-8
- Empirical Formula: C8H12O6
- Molecular Weight: 204.18
- MDL number: MFCD09261382
- EINECS: 617-849-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-11 16:58:07
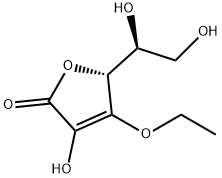
What is 3-O-Ethyl-L-ascorbic acid?
Description
3-O-Ethyl ascorbic acid ether, also called VC ethyl ether, is an ascorbic acid derivative. Ascorbic acid is a naturally occurring antioxidant and free radical scavenger, a reducing agent in the conversion process of biological enzymes, and has a preventive effect on some chronic diseases (cancer, diabetes and allergic skin diseases). From the molecular structure of ascorbic acid, it can be seen that compared to the 2-position enol hydroxyl group, the 3-position enol hydroxyl group has higher activity on electrophiles, and it is the product of 3-O-alkylation during O-alkylation modification. Mainly. 3-O-Ethyl ascorbate ether is a product of ascorbic acid-3-O-ethylation. It has a simple molecular structure and is easily absorbed by the skin. After entering the dermis through the stratum corneum of the skin, it is easily decomposed by the biological enzymes in the human body. The effect of ascorbic acid effectively solves the fat solubility problem of ascorbic acid, and is widely used in cosmetics as a whitening agent and antioxidant.
The Uses of 3-O-Ethyl-L-ascorbic acid
Vitamin C ethyl ether (VC ethyl ether) is a lipophilic and hydrophilic amphoteric vitamin C derivative. It not only retains the redox effect of vitamin C but is also very stable. It is a non-discoloring vitamin C derivative. It is a lipophilic and chemicalbook amphoteric substance, which not only makes it extremely convenient to use in the formula, but also makes it easy to penetrate the stratum corneum into the dermis. After entering the skin, it is easily decomposed by biological enzymes to play the role of vitamin C, thereby Improve its bioavailability.
The Uses of 3-O-Ethyl-L-ascorbic acid
3-O-Ethyl-L-ascorbic Acid could be a useful stabilizing agent for a para-hydroxyacetophenone solution. 3-O-Ethyl-L-ascorbic Acid could be a useful stabilizing agent for a para-?hydroxyacetophenone solution.
Definition
ChEBI: 3-O-ethyl-l-ascorbic acid (EA) is a butenolide. It is an l-ascorbic acid derivative with an ethyl group at the third carbon position.
Health effects
3-O-ethyl-L-ascorbic acid improves the molecules' stability and its transportation efficacy to the skin, being absorbed faster and better. Additionally, it is less irritating to the skin. Cosmetic case studies demonstrate that they have skin brightening, collagen stimulating, and antioxidant properties.
Mechanism of action
VC ethyl ether penetrates the stratum corneum to reach the melanocytes in the basal layer, inhibits tyrosinase activity, inhibits the formation of melanin, and restores melanin to colorless, thereby effectively whitening the skin. And Chemicalbook and VC ethyl ether can directly participate in the synthesis of collagen and repair skin cell activity after entering the dermis, increasing collagen, so that the skin becomes full and elastic, making the skin delicate and smooth.
Synthesis

The above 3-O-ethyl-5,6-O-isopropylideneascorbic acid 40g dissolved in 200ml of ethanol. 10g of cation-exchange resin was added and was refluxed for 2 hours. The catalyst was removed by an ion exchange resin and concentrated under reduced pressure. After drying, with ethyl acetate: hexane (3: 1) crystallization. After drying, obtained 33.3g 3-O-ethylascorbic acid, yield 94%. It was recrystallized in 100mL ethyl acetate giving 30.6g, yield 87%, mp: 112-114??C.
Properties of 3-O-Ethyl-L-ascorbic acid
| Melting point: | 112.0 to 116.0 °C |
| Boiling point: | 551.5±50.0 °C(Predicted) |
| Density | 1.46±0.1 g/cm3(Predicted) |
| vapor pressure | 0-0Pa at 20-25℃ |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| pka | 8.89±0.40(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C8H12O6/c1-2-13-7-5(11)8(12)14-6(7)4(10)3-9/h4,6,9-11H,2-3H2,1H3/t4-,6+/m0/s1 |
Safety information for 3-O-Ethyl-L-ascorbic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-O-Ethyl-L-ascorbic acid
| InChIKey | ZGSCRDSBTNQPMS-UJURSFKZSA-N |
| SMILES | O(CC)C1=C(O)C(=O)O[C@@H]1[C@H](CO)O |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 3-O-Ethyl-L-ascorbic Acid CAS 86404-04-8View Details
3-O-Ethyl-L-ascorbic Acid CAS 86404-04-8View Details
86404-04-8 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1