Riboflavin
Synonym(s):Riboflavin;Vitamin B2;Riboflavine;(?)-Riboflavin;Lactoflavin
- CAS NO.:83-88-5
- Empirical Formula: C17H20N4O6
- Molecular Weight: 376.36
- MDL number: MFCD00005022
- EINECS: 201-507-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
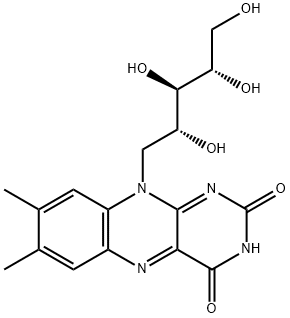
What is Riboflavin?
Absorption
Vitamin B2 is readily absorbed from the upper gastrointestinal tract.
Description
Riboflavin, also known as vitamin B2, is an enzyme cofactor in many flavoprotein enzyme reactions, notably the activation of other vitamins. It is widely present in nature, found in dairy products, eggs, organ tissues, and leafy vegetables, with yeast and its extracts being the richest natural source. Riboflavin was first synthesized in Germany and Austria in the 1930s by chemists H. Meerwein, R. Kuhn, and their colleagues. Initially, it was chemically manufactured for use as a nutritional supplement, primarily from o-xylene, D-ribose, and alloxan. Today, it is biosynthesized using fermenting organisms such as the fungus Ashbya gossypii and the bacterium Bacillus subtilis. This year, J. B. Metternich and R. Gilmour at the University of Munster (Germany) discovered a new use for riboflavin. In their search for a better way to make Z-olefins, they found that riboflavin can serve as a photocatalytic chromophore to isomerize the E-isomer of retinal (a vitamin A aldehyde found in retinas) to its Z-form. They used riboflavin to catalyze the selective, irreversible conversion of planar, conjugated E-olefins to their twisted, nonconjugated Z-counterparts. The researchers believe this reaction will be useful in synthesizing complex molecules such as drugs and agrochemicals.
Description
A water-soluble B fraction was found in the 1920s to contain a yellow, fluorescent growth factor called riboflavin in England and vitamin G in the United States. In the early 1930s, several groups found the coenzyme forms of riboflavin 50-phosphate (flavin mononucleotide) and the further conjugate with adenylic acid (flavin adenine dinucleotide).
Chemical properties
Yellow to orange/yellow crystalline powd
Chemical properties
VITAMIN B2 (Riboflavin). Some earlier designations for this substance included vitamin G, lactoflavin, hepatoflavin, ovoflavin, verdoflavin. The chemical name is 6,7-dimethyl-9-d-l’ribityl isolloxazine. Riboflavin is a complex pigment with a green fluorescence.
Physical properties
Riboflavin is moderately soluble in water (10–13 mg/dl) and ethanol but insoluble
in ether, chloroform, and acetone. It is soluble but unstable under alkaline
conditions.
The catalytic functions of riboflavin are carried out primarily at positions N-1,
N-5, and C-4 of the isoalloxazine nucleus. In addition, the methyl group at C-8
participates in covalent bonding with enzyme proteins. The flavin coenzymes are
highly versatile redox cofactors because they can participate in either one- or two electron redox reactions
Riboflavin antagonists include analogs of the isoalloxazine ring (e.g., diethylri boflavin, dichlororiboflavin) and the ribityl side chain (e.g., d-araboflavin,
d-galactoflavin, 7-ethylriboflavin).
Originator
Hyflavin ,Endo,US,1948
Occurrence
(-)-Riboflavin is a nutritional factor found in milk, eggs, malted barley, liver, kidney, heart, leafy vegetables. Richest natural source is yeast. Minute amounts present in all plant and animal cells. Vitamin (enzyme cofactor).
The Uses of Riboflavin
riboflavin (Vitamin B2) is used in skin care preparations as an emollient. It can be found in sun care products as a suntan enhancer. Medicinally, it is used for the treatment of skin lesions.
The Uses of Riboflavin
Nutritional factor found in milk, eggs, malted barley, liver, kidney, heart, leafy vegetables. Richest natural source is yeast. Minute amounts present in all plant and animal cells. Vitamin (enzyme cofactor).
The Uses of Riboflavin
Vitamin B2; Vitamin cofactor; LD50(rat) 560 mg/kg ip
The Uses of Riboflavin
Riboflavin is the water-soluble vitamin b2 required for healthy skin and the building and maintaining of body tissues. it is a yellow to orange-yellow crystalline powder. it acts as a coenzyme and carrier of hydrogen. it is stable to heat but may dissolve and be lost in cooking water. it is relatively stable to storage. sources include leafy vegetables, cheese, eggs, and milk.
The Uses of Riboflavin
Vitamin B2 (riboflavin) is produced by yeast from glucose, urea, and mineral salts in an aerobic fermentation.
Indications
For the treatment of ariboflavinosis (vitamin B2 deficiency).
Background
Nutritional factor found in milk, eggs, malted barley, liver, kidney, heart, and leafy vegetables. The richest natural source is yeast. It occurs in the free form only in the retina of the eye, in whey, and in urine; its principal forms in tissues and cells are as flavin mononucleotide and flavin-adenine dinucleotide.
Definition
ChEBI: D-Ribitol in which the hydroxy group at position 5 is substituted by a 7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl moiety. It is a nutritional factor found in milk, eggs, malted barley, liver, kidney, heart, and leafy vege ables, but the richest natural source is yeast. The free form occurs only in the retina of the eye, in whey, and in urine; its principal forms in tissues and cells are as flavin mononucleotide and flavin-adenine dinucleotide.
Manufacturing Process
100 g of riboflavin and 3 of potassium carbonate are suspended in 500 cc of the aqueous formaldehyde solution and the mixture is stirred at 30°C for 8 hours. At the end of this period, 5 cc of glacial acetic acid and 1 liter of methanol are added, with stirring. The solution is freed from undissolved material by filtration and the clear solution is poured slowly at about 20°C to 22°C with vigorous stirring into 8 liters of anhydrous acetone. The resultant precipitate is filtered off, washed repeatedly with anhydrous acetone and with ether, and then dried at room temperature and with vacuum. The resultant dried powder is dissolved in hot water at 95°C to give an aqueous solution of 20% by weight. This solution is kept in the dark at room temperature for 3 to 4 weeks, after which time a large amount of material crystallizes out of the solution. This crystallized material is removed by filtration and recrystallized from hot water. A small amount of dark red insoluble material is filtered from the hot solution. This recrystallization step is repeated four times. The resultant end product is monomethylol riboflavin, which crystallized in small orange clusters. It has a melting point of 232°C to 234°C with decomposition, and it becomes dark when heated above 225°C.
brand name
Flavaxin (Sterling Winthrop).
Therapeutic Function
Enzyme cofactor vitamin source
General Description
The conflicting results were eventually found to be due,in part, to deficiencies in study animals not just of vitamin B2,but also vitamin B3 (niacin), the cause of human forms of pellagra,and/or vitamin B6 (pyridoxine), another cause of dermatitis.Likewise, treatments with vitamin B2 were inconsistentbecause the early sources of this vitamin contained otherB vitamins. Vitamin B2 was eventually isolated from eggwhites in 1933 and produced synthetically in 1935. Thename riboflavine was officially accepted in 1960; althoughthe term was in common use before then. In 1966, IUPACchanged it to riboflavin, which is in common use today.Riboflavin is synthesized by all green plants and by mostbacteria and fungi. Therefore, riboflavin is found, at least insmall amounts, in most foods. Foods that are naturally highin riboflavin include milk and other dairy products, meat,eggs, fatty fish, and dark green vegetables.
Chemically, riboflavin is an N-glycoside of flavin, alsoknown as lumichrome, and the sugar, ribitol .Flavin is derived from the Latin word flavus for “yellow”because of the yellow color of its crystals and yellow fluorescenceunder UV light. Riboflavin is heat stable but easilydegraded by light. Its systematic names are 7,8-dimethyl-10-ribitylisoalloxazine and 7,8-dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)isoalloxazine.
Biochem/physiol Actions
Riboflavin serves as a precursor for the active enzyme cofactors riboflavin 5′-monophosphate (also called flavin mononucleotide or FMN) and flavin adenine dinucleotide (FAD). Riboflavin deficiency in the diet results in a well-defined syndrome known as ariboflavinosis, Riboflavin exhibits protective effects against tumor development and cardiovascular disease. Its deficiency often affects metabolism involving redox reactions. Riboflavin is found essential for iron absorption, gastrointestinal development, neurogenesis, corneal vascularization and corneal opacity.
Pharmacokinetics
Riboflavin, or vitamin B2, is an easily absorbed, water-soluble micronutrient crucial for maintaining human health. Like other B vitamins, it supports energy production by aiding in the metabolism of fats, carbohydrates, and proteins. Vitamin B2 is necessary for red blood cell formation and respiration, antibody production, and the regulation of human growth and reproduction. It is essential for healthy skin, nails, and hair growth, as well as overall good health, including the regulation of thyroid activity. Additionally, riboflavin helps prevent or treat various eye disorders, including some cases of cataracts.
Clinical Use
Severe riboflavin deficiency is known as ariboflavinosis, andtreatment or prevention of this condition is the only provenuse of riboflavin. Ariboflavinosis is most commonly associatedwith multiple vitamin deficiency as a result of alcoholismin developed countries. Because of the large numberof enzymes requiring riboflavin as a coenzyme, deficienciescan lead to a wide range of abnormalities. In adults seborrheicdermatitis, photophobia, peripheral neuropathy, anemia, andoropharyngeal changes including angular stomatitis, glossitis,and cheilosis, are often the first signs of riboflavin deficiency.In children, cessation of growth can also occur. As the deficiencyprogresses, more severe pathologies develop untildeath ensues. Riboflavin deficiency may also produce teratogeniceffects and alter iron handling leading to anemia.
Safety Profile
Poison by intravenous route. Moderately toxic by intraperitoneal and subcutaneous routes. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Environmental Fate
Physicochemical Properties
Riboflavin has the appearance of a yellow to orange amorphous
solid and imparts an orange color to the B vitamin tablets. Riboflavin has a melting point of 290°C, a density of
1.65, and a refractive index of 135°. The pKa is 9.888 and log P
is 0.095. Riboflavin has solubility in water of 0.1 g l-1.
Exposure Routes and Pathways
The route of exposure is oral. Dietary sources of riboflavin and
its coenzymes include broccoli, spinach, asparagus, enriched
flour, yeast, eggs, milk, cheese, mackerel, trout, poultry, liver,
and kidneys.
Toxicokinetics
Riboflavin, which is only moderately water soluble, is absorbed
from the gastrointestinal tract but is limited to about 27 mg at
any one time from an oral dose given to an adult. Hence, mega
doses would not be expected to increase significantly the total
amount absorbed. It is hepatically metabolized, protein
bound, and widely distributed to tissue; however, little is stored
in the liver, spleen, heart, and kidneys. Riboflavin is excreted
renally as metabolites, which have been oxidatively cleaved in
the ribityl side chain and converted to hydroxymethyls in the
ring methyl functions. Riboflavin in excess of daily body needs
is excreted unchanged in the urine. Riboflavin exhibits biphasic
pharmacokinetics with initial and terminal half-lives of 1.4 and
14 h, respectively.
Metabolism
Hepatic.
Purification Methods
It crystallises from H2O as a yellow-orange powder in three different forms with differing amounts of H2O. It melts if placed in an oil bath at 250o, but decomposes at 280o if heated at a rate of 5o/minute. It is also purified by crystallisation from 2M acetic acid, then extracted with CHCl3 to remove lumichrome impurity. [Smith & Metzler J Am Chem Soc 85 3285 1963.] Its solubility in H2O is 1g in 3-15L depending on the crystal structure. Its solubility in EtOH at 25o is 4.5mg in 100mL. Store it in the dark because it is decomposed to lumichrome by UV light. [Pearson The Vitamins vol V pp1-96 1967 and vol VII pp 1-96 1972, Gy.gy and Pearson eds, Academic Press, Beilstein 26 IV 2542.]
Properties and Applications
yellow
Properties of Riboflavin
| Melting point: | 290 °C (dec.)(lit.) |
| Boiling point: | 504.93°C (rough estimate) |
| alpha | -135 º (c=5, 0.05 M NaOH) |
| Density | 1.2112 (rough estimate) |
| refractive index | -135 ° (C=0.5, JP Method) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Very slightly soluble in water, practically insoluble in ethanol (96 per cent). Solutions deteriorate on exposure to light, especially in the presence of alkali. It shows polymorphism (5.9). |
| form | Powder |
| pka | 1.7(at 25℃) |
| color | Yellow to orange |
| Odor | Slight odour |
| PH | 5.5-7.2 (0.07g/l, H2O, 20°C) |
| PH Range | 6 |
| Water Solubility | 0.07 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,8200 |
| BRN | 97825 |
| Stability: | Stable, but light-sensitive. Incompatible with strong oxidizing agents, reducing agents, bases, calcium, metallic salts. May be moisture sensitive. |
| CAS DataBase Reference | 83-88-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Riboflavine(83-88-5) |
| EPA Substance Registry System | Riboflavin (83-88-5) |
Safety information for Riboflavin
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Riboflavin
| InChIKey | AUNGANRZJHBGPY-SCRDCRAPSA-N |
Riboflavin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Vitamin B2 99%View Details
Vitamin B2 99%View Details -
 Riboflavin 98% CAS 83-88-5View Details
Riboflavin 98% CAS 83-88-5View Details
83-88-5 -
 Riboflavin CAS 83-88-5View Details
Riboflavin CAS 83-88-5View Details
83-88-5 -
 Riboflavin for cell culture CAS 83-88-5View Details
Riboflavin for cell culture CAS 83-88-5View Details
83-88-5 -
 Vitamin B2 PowderView Details
Vitamin B2 PowderView Details
83-88-5 -
 Riboflavin IHRSView Details
Riboflavin IHRSView Details
83-88-5 -
 Vitamin B2 PowderView Details
Vitamin B2 PowderView Details
130-40-5 -
 Vitamin B2 powderView Details
Vitamin B2 powderView Details
83-88-5
