2,3-Diaminobenzoic acid
- CAS NO.:603-81-6
- Empirical Formula: C7H8N2O2
- Molecular Weight: 152.15
- MDL number: MFCD00137818
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-25 16:21:11
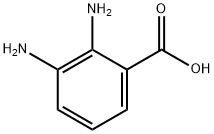
What is 2,3-Diaminobenzoic acid?
The Uses of 2,3-Diaminobenzoic acid
2,3-Diaminobenzoic acid (23DBA) is an important organic reagent mainly used in scientific research and laboratory experiments. 23DBA can undergo polycondensation with terephthalic acid dichloro anhydride[1]. 23DBA isomer is also used in the study of luminescence mechanism of red luminescent carbon dots (RCDs)[2]. In addition, 2,3-Diaminobenzoic acid also possesses antioxidant properties that help reduce the formation of free radicals in the body. It is also used as a chelating agent that effectively binds to metal ions and prevents them from forming insoluble complexes.
What are the applications of Application
2,3-Diaminobenzoic acid is an aromatic building block
Synthesis
2,3-Diaminobenzoic acid is synthesised using 3-nitroanthranilic acid as a raw material by chemical reaction. The specific synthesis steps are as follows:
2-amino-3-nitrobenzoic acid (9.36 g, 51.4 mmol) was dissolved in MeOH (100 mL) and there was added 10% Pd/C (2.0g). The reaction was stirred under H2-atmosphere (ambient pressure) until absorption ceased. The reaction was filtered through a pad of Celite and the filtrate was evaporated under reduced pressure. The product was purified by flash chromatography (CH2Cl2/MeOH 5:1) and the collected fractions were evaporated to give 2,3-Diaminobenzoic acid (5.40 g, 69%) as a dark brown solid. mp. 199 °C dec. (Litt4. 201 °C dec.). 1H NMR (300 MHz, DMSO-d6) δ 7.09 (dd, J = 8.0, 1.5 Hz, 1H), 6.67 (dd, J = 7.4, 1.5 Hz, 1H), 6.34 (dd, J = 8.0, 7.4 Hz, 2H), 6.32 (br s, 4H). 13C NMR (75 MHz, DMSO-d6) δ 170.5, 139.6, 135.6, 119.4, 117.0, 114.9, 110.3.
References
[1] V. N. ODNORALOVA. The polycondensation reaction of 2,4-diaminobenzoic acid with dichloroanhydride of terephthalic acid[J]. Fibre Chemistry, 1974. DOI:10.1007/BF00542946.
[2] LIU Y, DING H, ZHANG S, et al. Photoluminescence mechanism of red emissive carbon dots from a diaminobenzoic acid isomer?[J]. Materials Advances, 2023. DOI:10.1039/D3MA00836C.
Properties of 2,3-Diaminobenzoic acid
| Melting point: | 198-204 °C |
| Boiling point: | 274.61°C (rough estimate) |
| Density | 1.2804 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| pka | 5.10±0.10(Predicted) |
| form | solid |
| color | Brown to dark brown |
| CAS DataBase Reference | 603-81-6(CAS DataBase Reference) |
Safety information for 2,3-Diaminobenzoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 2,3-Diaminobenzoic acid
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1