2,2-Dimethylbutane
Synonym(s):Neohexane
- CAS NO.:75-83-2
- Empirical Formula: C6H14
- Molecular Weight: 86.18
- MDL number: MFCD00009321
- EINECS: 200-906-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
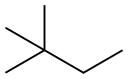
What is 2,2-Dimethylbutane?
Chemical properties
2,2-dimethylbutane is the chain isomeric form of hexane. It is a colorless and flammable liquid with a specific gravity of 0.6444. Insoluble in water, miscible with alcohol, ether, acetone, benzene and petroleum ether. The solubility is similar to that of hexane and 2-methylpentane.
Physical properties
Clear, colorless liquid with a mild petroleum-like odor. Liquid quickly evaporates and forms conbustible fumes. An odor threshold concentration of 20 ppmv was reported by Nagata and Takeuchi (1990).
The Uses of 2,2-Dimethylbutane
2,2-Dimethylbutane is an acyclic alkane. Its Research Octane Number (RON) has been reported to be 91.8. It is used to Component of high-octane motor and aviation fuels, intermediate for agricultural chemicals.
Production Methods
2,2-Dimethylbutane is produced from crude oil, natural liquid gases, and petroleum refining processes.
What are the applications of Application
2,2-Dimethylbutane may be employed as probe to investigate the nature of possible active sites in supported metal catalysts.
Some of the reported applications of 2,2-dimethylbutane include:
Palladium-catalyzed radical oxidative alkoxycarbonylation to prepare various alkyl carboxylates.
Iridium-iron-catalyzed tandem dehydrogenation-isomerization-hydrosilylation to synthesize corresponding silane.
Preparation
2,2-Dimethylbutane can be synthesised by the hydroisomerisation of 2,3-dimethylbutane using an acid catalyst.
By the thermal or catalytic union (alkylation) of ethylene and isobutane, both recovered from refinery gases.
General Description
Colorless liquid with an odor of gasoline. Less dense than water and insoluble in water. Hence floats on water. Irritating vapor. Flash point -54°F.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
2,2-Dimethylbutane may be incompatible with strong oxidizing agents like nitric acid. Charring may occur followed by ignition of unreacted material and other nearby combustibles. In other settings, mostly unreactive. Not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. When heated sufficiently or when ignited in the presence of air, oxygen or strong oxidizing agents, burns exothermically to produce carbon dioxide and water.
Hazard
Highly flammable, dangerous fire and explosion risk, explosion limits 1.2–7%
Health Hazard
Inhalation causes dizziness, nausea, and vomiting; concentrated vapor may cause unconsciousness and collapse. Contact with liquid causes irritation of eyes; repeated contact may produce irritation of skin. Ingestion causes irritation of stomach. Aspiration causes severe lung irritation, rapidly developing pulmonary edema, and central nervous system excitement followed by depression.
Safety Profile
Probably an irritant and narcotic in high concentration. A very dangerous fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidzing materials. Keep away from heat or open flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Environmental Fate
Photolytic. Reported photooxidation rate constants for the reaction of 2,2-dimethylbutane with
OH radicals at 297, 299, and 300 K are 2.59 x 10-12, 6.16 x 10-12, and 2.59 x 10-12
cm3/molecule?sec, respectively (Atkinson, 1985, 1990).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water vapor. 2,2-
Dimethylbutane will not hydrolyze because it has no hydrolyzable functional group.
Purification Methods
Distil it azeotropically with MeOH, then wash it with water, dry (Na2SO4) it, and distil it. [Beilstein 1 IV 367.]
Properties of 2,2-Dimethylbutane
| Melting point: | −100 °C(lit.) |
| Boiling point: | 50 °C(lit.) |
| Density | 0.649 g/mL at 25 °C(lit.) |
| vapor density | 2.97 (vs air) |
| vapor pressure | 5.35 psi ( 20 °C) |
| refractive index | n |
| Flash point: | <−30 °F |
| storage temp. | Flammables area |
| solubility | In methanol: 590 and 800 g/L at 5 and 10 °C, respectively. Miscible at higher temperatures (Kiser
et al., 1961). Miscible with other aliphatic hydrocarbons, e.g., pentane, hexane, heptane, etc. |
| form | Liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| color | Clear colorless to slightly yellow |
| explosive limit | ~7.7% |
| Odor Threshold | 20ppm |
| Water Solubility | insoluble |
| BRN | 1730736 |
| Henry's Law Constant | 1.69(atm?m3/mol) at 25 °C (Mackay and Shiu, 1981) |
| Dielectric constant | 1.8700000000000001 |
| Exposure limits | ACGIH TLV: TWA and STEL for all isomers except n-hexane are 500 and
1,000 ppm, respectively (adopted). |
| Stability: | Stable, but may be moisture sensitive. Highly flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 75-83-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Butane, 2,2-dimethyl-(75-83-2) |
| EPA Substance Registry System | 2,2-Dimethylbutane (75-83-2) |
Safety information for 2,2-Dimethylbutane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for 2,2-Dimethylbutane
2,2-Dimethylbutane manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2,2-Dimethylbutane CAS 75-83-2View Details
2,2-Dimethylbutane CAS 75-83-2View Details
75-83-2 -
 2,2-Dimethylbutane CAS 75-83-2View Details
2,2-Dimethylbutane CAS 75-83-2View Details
75-83-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8