2'-Deoxyinosine
Synonym(s):9-(2-Deoxy-β-D -ribofuranosyl)hypoxanthine
- CAS NO.:890-38-0
- Empirical Formula: C10H12N4O4
- Molecular Weight: 252.23
- MDL number: MFCD00005762
- EINECS: 212-964-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
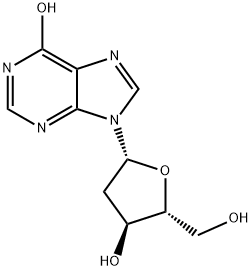
What is 2'-Deoxyinosine?
Chemical properties
Crystalline
The Uses of 2'-Deoxyinosine
An Inosine (I661000) analog with hypotensive activity. An impurity of the antiviral drug 2’,3’-Dideoxyinosine (D440950).
The Uses of 2'-Deoxyinosine
2′-Deoxyinosine has been used in the quantification of nucleoside forms of DNA lesion in a single DNA sample by liquid chromatography tandem mass spectrometry (LC-MS/MS). It has also been used as a standard for high performance liquid chromatography analysis.
What are the applications of Application
2′-Deoxyinosine is a nucleoside composed of hypoxanthine attached to 2′-deoxyribose via a β-N9-glycosidic bond
Definition
ChEBI: 2'-deoxyinosine is a purine 2'-deoxyribonucleoside that is inosine in which the hydroxy group at position 2' is replaced by a hydrogen. It has a role as a Saccharomyces cerevisiae metabolite, a human metabolite, a plant metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a purines 2'-deoxy-D-ribonucleoside and a purine 2'-deoxyribonucleoside. It is functionally related to an inosine.
Biochem/physiol Actions
2′-Deoxyinosine is a nucleoside form of hypoxanthine. It is a DNA damage product resulting from the impairment of DNA by reactive nitrogen species. 2′-deoxyinosine is formed from nitrosative deamination by N2O3.
Purification Methods
2'-Deoxyinosine [890-38-0] M 252.2, m 206o(dec), 218-220o(dec), [ ] D25 -21o (c 2, N ] D -21o (c 1, H 2O), pKEst(1) ~ 8.9, pKEst(2) ~ 12.4. Purify 2'-deoxyinosine by recrystallisation from H2O. [Brown & Lythgoe J Chem Soc 1990 1950, UV: MacNutt Biochem J 50 384 1952, Beilstein 26 III/IV 2086.]
Properties of 2'-Deoxyinosine
| Melting point: | 250 °C |
| Boiling point: | 395.4°C (rough estimate) |
| alpha | 21 º (c=1, H2O 22 ºC) |
| Density | 1.3402 (rough estimate) |
| refractive index | -16 ° (C=1, H2O) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Water (Slightly) |
| pka | 8.92±0.70(Predicted) |
| form | Powder |
| color | White to Off-white |
| Water Solubility | 8.33g/L(25.02 ºC) |
| BRN | 33517 |
| InChI | InChI=1S/C10H12N4O4/c15-2-6-5(16)1-7(18-6)14-4-13-8-9(14)11-3-12-10(8)17/h3-7,15-16H,1-2H2,(H,11,12,17)/t5-,6+,7+/m0/s1 |
| CAS DataBase Reference | 890-38-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Inosine, 2'-deoxy-(890-38-0) |
Safety information for 2'-Deoxyinosine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2'-Deoxyinosine
| InChIKey | VGONTNSXDCQUGY-RRKCRQDMSA-N |
| SMILES | OC[C@H]1O[C@@H](N2C3C(=C(N=CN=3)O)N=C2)C[C@@H]1O |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
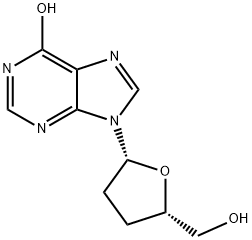




You may like
-
 2'-Deoxyinosine CAS 890-38-0View Details
2'-Deoxyinosine CAS 890-38-0View Details
890-38-0 -
 2′-Deoxyinosine CAS 890-38-0View Details
2′-Deoxyinosine CAS 890-38-0View Details
890-38-0 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
