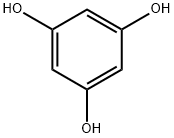
1,2,4-Benzenetriol
Synonym(s):1,2,4-Trihydroxybenzene;2-Heptyl-4-quinolone;Hydroxyhydroquinone
- CAS NO.:533-73-3
- Empirical Formula: C6H6O3
- Molecular Weight: 126.11
- MDL number: MFCD00002198
- EINECS: 208-575-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
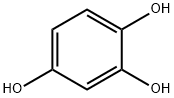
What is 1,2,4-Benzenetriol?
Chemical properties
Gray powder
Physical properties
1,2,4-Benzenetriol forms colorless platelets or prisms that quickly colorize in air. It is soluble in water and polar solvents, and slightly soluble in chloroform and carbon disulfide.
The Uses of 1,2,4-Benzenetriol
A metabolite of benzene.
The Uses of 1,2,4-Benzenetriol
1,2,4-Trihydroxybenzene is an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff.It is used as a metabolite of benzene.
Definition
ChEBI: Benzene-1,2,4-triol is a benzenetriol carrying hydroxy groups at positions 1, 2 and 4. It has a role as a mouse metabolite.
What are the applications of Application
1,2,4-Benzenetriol is a metabolite of benzene
Production Methods
1,2,4-Benzenetriol is synthesized by hydrolysis of 1,2,4-triacetoxybenzene, which is prepared by the acid-catalyzed
reaction of p-benzoquinone with acetic anhydride.
Other production methods for 1,2,4-Benzenetriol are oxidation of resorcinol with hydrogen peroxide and Dakin oxidation of 2,4- or
3,4-dihydroxybenzaldehydes or 2,4- or 3,4-dihydroxyacetophenones with alkaline hydrogen peroxide solution.
General Description
1,2,4-Benzenetriol is also known as hydroxyhydroquinone. It is an intermediary metabolite of benzene that is present in roasted coffee beans. It is mutagenic and it causes cleaving of DNA single strands by the generation of reactive oxygen species.
Chemical Reactivity
1,2,4-Benzenetriol is, like other phenols, a strong reducing agent. Its basic aqueous solution absorbs gaseous oxygen to produce black precipitates of the humic acid type. 1,2,4-Benzenetriol shows typical reactions of phenols. Derivatives of the tautomeric keto form are also known: halogenation of hydroxyhydroquinone, for example, gives 1,2,5,5-tetrahalocyclohexene-3,4,6-trione. Condensation with ethylacetoacetate produces dihydroxycoumarin; reaction with phthalic anhydride yields hydroxyhydroquinonephthalein. Monosubstitution of a hydroxy group by an amino group occurs easily at room temperature and gives 2,4-dihydroxyaniline.
Safety Profile
Poison by subcutaneous and intraperitoneal routes. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Crystallise the triol from Et2O or Et2O/EtOH, and dry it in a vacuum. The picrate forms orange-red needles m 96o. [Beilstein 6 H 1087, 6 I 541, 6 II 1071, 6 III 6276.]
Properties of 1,2,4-Benzenetriol
| Melting point: | 140 °C (subl.) (lit.) |
| Boiling point: | 194.21°C (rough estimate) |
| Density | 1.45 g/cm3 (20℃) |
| refractive index | 1.5627 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO (Sparingly), Methanol (Slightly) |
| form | Solid |
| pka | 9.58±0.10(Predicted) |
| color | Plates from Et2O |
| Water Solubility | freely soluble |
| Sensitive | Air Sensitive |
| Merck | 14,1073 |
| BRN | 2042863 |
| Stability: | Light Sensitive, Moisture Sensitive |
| CAS DataBase Reference | 533-73-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2,4-Benzenetriol(533-73-3) |
| EPA Substance Registry System | 1,2,4-Benzenetriol (533-73-3) |
Safety information for 1,2,4-Benzenetriol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for 1,2,4-Benzenetriol
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 1,2,4-Trihydroxybenzene CAS 533-73-3View Details
1,2,4-Trihydroxybenzene CAS 533-73-3View Details
533-73-3 -
 1,2,4-Trihydroxybenzene CAS 533-73-3View Details
1,2,4-Trihydroxybenzene CAS 533-73-3View Details
533-73-3 -
 1,2,4-Benzenetriol CAS 533-73-3View Details
1,2,4-Benzenetriol CAS 533-73-3View Details
533-73-3 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0