3-Hydroxybenzoic acid
Synonym(s):m-Salicylic acid;3-Hydroxybenzoic acid
- CAS NO.:99-06-9
- Empirical Formula: C7H6O3
- Molecular Weight: 138.12
- MDL number: MFCD00002506
- EINECS: 202-726-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
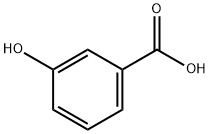
What is 3-Hydroxybenzoic acid?
Chemical properties
White to light yellow crystal powder. 3-Hydroxybenzoic acid does not undergo decarboxylation at elevated temperature and is stable up to 300℃. This is in contrast to 2- and 4-hydroxybenzoic acids. Electrophilic substitution (e.g., nitration, halogenation, sulfonation) occurs ortho or para to the hydroxyl group. Thus, nitration with 62 % aqueous nitric acid gives 2-nitro-3-hydroxybenzoic acid as the main product, along with 4-nitro- and 6-nitro-3-hydroxybenzoic acids.
The Uses of 3-Hydroxybenzoic acid
3-Hydroxybenzoic acid is used as an intermediate in the manufacture of germicides, plasticizers, preservatives, light stabiliziers, petroleum additives, pharmaceuticals, and plasticizer. It can also be used for the synthesis of azo dyes and herbicide fomesafen.
Definition
ChEBI: 3-hydroxybenzoic acid is a monohydroxybenzoic acid that is benzoic acid substituted by a hydroxy group at position 3. It has been isolated from Taxus baccata. It is used as an intermediate in the synthesis of plasticisers, resins, pharmaceuticals, etc. It has a role as a bacterial metabolite and a plant metabolite. It derives from a benzoic acid. It is a conjugate acid of a 3-hydroxybenzoate.
What are the applications of Application
3-Hydroxybenzoic acid is used in perfumery. It is an important raw material and intermediate used in organic synthesis, pharmaceuticals agrochemicals and dyestuff fields.
Preparation
3-Hydroxybenzoic acid is synthesized from benzoic acid by sulfonation, alkali melting and hydrolysis.
122kg of benzoic acid was put into the reactor, and 100kg of oleum was added under stirring. The temperature was raised to about 100 °C, and the reaction was carried out for 2h to obtain Sodium 3-sulfobenzoate. Then the sulfonated liquid was transferred to an alkali melting pot, 45 kg of solid sodium hydroxide was added, the temperature was raised to melting, and reacted for 4 to 5 hours to obtain sodium m-carboxyphenate, and diluted sulfuric acid was added for acid hydrolysis. Cool and crystallize, filter out sodium sulfate, and decolorize the filter cake with activated carbon to obtain 3-Hydroxybenzoic acid.
Reactions
m-Hydroxybenzoic Acid, of the three hydroxybenzoic acids, the metaisomer is of least commercial importance. Unlike salicylic acid, m-hydroxybenzoic acid does not undergo the Friedel-Crafts reaction. It can be converted in 80% yield to m-aminophenol by the Schmidt reaction, which involves treating the acid with hydrazoic acid in trichloroethylene in the presence of sulfuric acid at 40 °C.
Synthesis Reference(s)
Tetrahedron Letters, 9, p. 3845, 1968 DOI: 10.1016/S0040-4039(01)99116-6
Purification Methods
Crystallise the hydroxyacid from absolute EtOH. [Beilstein 10 IV 315.]
Properties of 3-Hydroxybenzoic acid
| Melting point: | 200-203 °C (lit.) |
| Boiling point: | 213.5°C (rough estimate) |
| Density | 1.4850 |
| refractive index | 1.4600 (estimate) |
| FEMA | 4431 | 3-HYDROXYBENZOIC ACID |
| Flash point: | 220 °C |
| storage temp. | Store below +30°C. |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 4.06(at 19℃) |
| form | Powder |
| color | White to off-white |
| PH | 3 (H2O)Aqueous solution |
| Water Solubility | slightly soluble |
| BRN | 508160 |
| CAS DataBase Reference | 99-06-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 3-hydroxy-(99-06-9) |
| EPA Substance Registry System | m-Hydroxybenzoic acid (99-06-9) |
Safety information for 3-Hydroxybenzoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Hydroxybenzoic acid
| InChIKey | IJFXRHURBJZNAO-UHFFFAOYSA-N |
3-Hydroxybenzoic acid manufacturer
Chemixl intermediates pvt Ltd
New Products
Tetrabutylammonium hydrogen sulfate 2,2,6-Trimethyl-4H-1,3-dioxin-4-one 4-Piperidinopiperidine tert-Butyl acetoacetate 4-BROMOMETHYLTETRAHYDROPYRAN 3-Hydroxyazetidine hydrochloride Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) 3-Methoxybenzonitrile 4-Methoxybenzonitrile Dibenzoyl Peroxide Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran


You may like
-
 99-06-9 3-HYDROXY BENZOIC ACID 99%View Details
99-06-9 3-HYDROXY BENZOIC ACID 99%View Details
99-06-9 -
 METAHYDROXY BENZOIC ACID 99%View Details
METAHYDROXY BENZOIC ACID 99%View Details -
 99-06-9 3-Hydroxybenzoic acid 98%View Details
99-06-9 3-Hydroxybenzoic acid 98%View Details
99-06-9 -
 m-Hydroxybenzoic acid CAS 99-06-9View Details
m-Hydroxybenzoic acid CAS 99-06-9View Details
99-06-9 -
 3 Hydroxy Benzoic Acid 98%View Details
3 Hydroxy Benzoic Acid 98%View Details
99-06-09 -
 3-Hydroxybenzoic acid 99%View Details
3-Hydroxybenzoic acid 99%View Details -
 3-Hydroxybenzoic Acid CAS 99-06-9View Details
3-Hydroxybenzoic Acid CAS 99-06-9View Details
99-06-9 -
 3-Hydroxybenzoic acid CAS 99-06-9View Details
3-Hydroxybenzoic acid CAS 99-06-9View Details
99-06-9
