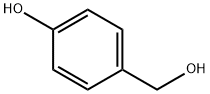
4-Methoxyphenol
Synonym(s):MEHQ;4-Methoxyphenol;Hydroquinone monomethyl ether;HQMME;4-MP
- CAS NO.:150-76-5
- Empirical Formula: C7H8O2
- Molecular Weight: 124.14
- MDL number: MFCD00002332
- EINECS: 205-769-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:05
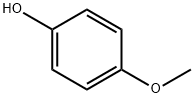
What is 4-Methoxyphenol?
Description
4-Methoxyphenol is a colorless to white, waxy solid with an odor of caramel and phenol. A combustible solid. Molecular weight= 124.15;Boiling point=243℃; Freezing/Melting point=5253℃; Flash point=132℃. Hazard Identification (based on NFPA-704 M Rating System): Health 2, Flammability 1, Reactivity 0. Slightly soluble in water.
Chemical properties
4-Methoxyphenol is a colorless to white, waxy solid with an odor of caramel and phenol. It is a combustible solid. It is used as an inhibitor for acrylic monomers and as a stabilizer for chlorinated hydrocarbons, ethyl cellulose, and UV inhibitors.
The Uses of 4-Methoxyphenol
4-methoxyphenol may be used in the synthesis of butylated hydroxy anisoles via alkylation with methyl tert-butyl ether over a non-zeolitic solid acidic catalyst. This process is eco-friendly when compared to the Friedel-Crafts alkylation reaction. 4-MP may also react with aqueous nitrous acid to form 2-nitro-4-methoxyphenol and benzoquinone in varying ratios depending on the reaction conditions. 4-MP can be used as a building block in designing β-cyclodextrin 4-methoxyphenol conjugates that can be potential ligands for drug complexation.
The Uses of 4-Methoxyphenol
4-Methoxyphenol is an active ingredient and used in dermatology. It is employed as a pharmaceutical drug in skin depigmentation. It is used as polymerization inhibitors. For example, in the radical polymerization of acryaltes and styrene monomers. It is also used as an intermediate in the preparationagrochemicals, liquid crystals. It acts as a stabilizer for the formulation of inks, toners and adhesives. It is mainly used as an additive for textile and leather industries.
Background
Mequinol is a phenol used in various applications. It is used as an inhibitor for acrylic monomers and acrylonitirles, as a stabilizer for chlorinated hydrocarbons and ethyl cellulose, as an ultraviolet inhibitor, as a chemical intermediate in the manufacture of antioxidants, pharmaceuticals, plasticizers, and dyestuffs . It is found as an active ingredient in topical drugs used for skin depigmentation indicated for the treatment of solar lentigines.
Indications
Mequinol is currently primarily available only as an active ingredient in combination products combined with tretinoin that are indicated for the treatment of solar lentigines and related hyperpigmented lesions resulting from chronic sun exposure .
Indications
Mequinol (4-hydroxyanisole) is a substrate of the enzyme tyrosinase and acts as a competitive inhibitor of melanogenesis.
Synthesis
4-Methoxyphenol was synthesised according to Oxidation with H2O2 and a Diselenide catalyst.
p-Anisaldehyde (50 mmol) is dissolved in CH2Cl2 (100mL) and (o-NO2PhSe)2 (2 mmol) and 30% H2O2 (13mL, 128 mmol) are added. The mixture is stirred magnetically at room temperature (water bath) for 30 minutes. Insoluble catalyst is removed by filtration and washed with CH2Cl2 (20mL) and water (20mL). It can be reused after drying. To the filtrate and washings, water (100mL) is added, and the layers are separated after shaking. The organic layer is washed subsequently with 10% NaHSO3 (100mL), 10% Na2CO3 (100mL), water (100mL) and dried over Na2SO4. 4-methoxyphenol is obtained by alkaline hydrolysis of the residue. Yield: 93%.
Definition
ChEBI: P-methoxyphenol is a member of phenols and a member of methoxybenzenes. It has a role as a metabolite.
Reactions
4-methoxyphenol(4-MP) may be used in the synthesis of butylated hydroxy anisoles via alkylation with methyl tert-butyl ether over a non-zeolitic solid acidic catalyst. This process is eco-friendly when compared to the Friedel-Crafts alkylation reaction. 4-MP may also react with aqueous nitrous acid to form 2-nitro-4-methoxyphenol and benzoquinone in varying ratios depending on the reaction conditions. 4-MP can be used as a building block in designing β-cyclodextrin 4-methoxyphenol conjugates that can be potential ligands for drug complexation.
Synthesis Reference(s)
The Journal of Organic Chemistry, 42, p. 1479, 1977 DOI: 10.1021/jo00428a054
Synthesis, p. 751, 1983 DOI: 10.1055/s-1983-30501
Tetrahedron Letters, 34, p. 7667, 1993 DOI: 10.1016/S0040-4039(00)61534-4
General Description
Pink crystals or white waxy solid.
Air & Water Reactions
Sensitive to moisture. Water soluble.
Reactivity Profile
4-Methoxyphenol can react with oxidizing materials.
Hazard
Eye irritant and skin damage.
Health Hazard
4-Methoxyphenol is expected to cause liver and renal toxicity with narcosis, but only at high levels of exposure.
Fire Hazard
Combustible
Flammability and Explosibility
Non flammable
Pharmacokinetics
Mequinol is in fact considered a melanocytotoxic chemical which when oxidized in melanocytes results in the formation of toxic entities like quinones . Such cytotoxic compounds subsequently have the potential to damage and destroy pigment cells, therefore causing skin depigmentation . In response, skin cells are naturally capable of protecting themselves against such cytotoxic agents with the help of endogenous intracellular glutathione and the detoxification action of glutathione S-transferase on the cytotoxic compounds . Regardless, it is consequently by way of this seemingly negative and damaging pharmacodynamic profile by which the mechanism of action of mequinol is sometimes described .
Safety Profile
Poison by intraperitoneal route. A skin irritant. When heated to decomposition it emits acrid smoke and fumes. See also ETHERS.
Potential Exposure
Mutagen, Primary Irritant. This compound is used in the manufacture of antioxidants, pharmaceuticals, plasticizers, and dyestuffs. It is used as a stabilizer and UV inhibitor in various polymers.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Absorption
The systemic exposure to mequinol was assessed in eight healthy subjects following two weeks of twice-daily topical treatment of a tretinoin and mequinol combination product . About dose of the product corresponding to about 37.3 ug/cm^2 of mequinol was applied to the subjects' backs . The mean Cmax for mequinol was 9.92 ng/mL (range between 4.22 and 23.62 ng/mL) and the Tmax was 2 hours (range between 1 to 2 hours) . The safety of mequinol in this combination formulation is supported by the low systemic exposures of the agent in the subjects .
Metabolism
Urine samples from melanoma patients treated with mequinol were analyzed and various mequinol metabolites were identified, including 3,4-dihydroxyanisole, the two o-methyl derivatives 3-hydroxy-4-methoxyanisole and 4-hydroxy-3-methoxyanisole, and even hydroquinone which may have originated at least partly from mequinol . All these identified metabolites were excreted predominantly as sulphates and glucuronides - only a small portion of the substances were present in urine in an unconjugated form . Ultimately, the 3,4-dihydroxyanisole is considered the most important metabolite of mequinol .
Toxicity
If a mequinol and tretinoin combination topical product is applied excessively, some potential adverse effects like marked redness, peeling of skin, discomfort, discoloration, or hypopigmentation may occur . Such products are not indicated for oral ingestion .
MSDS data reports that chronic exposure to a dose of 25 UMOL/L in humans can result in mutagenic effects like DNA inhibition in lymphocytes .
Storage
Protect containers from physical damage. Store in tightly closed containers in a cool, well-ventilated area. Sources of ignition, such as smoking and open flames, are prohibited where 4-methoxyphenol is used, handled, or stored in a manner that could create a potential fire or explosion hazard.
Shipping
UN3335 Aviation regulated solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Crystallise 4-methoxyphenol from *benzene, pet ether or H2O, and dry it under vacuum over P2O5 at room temperature. Sublime it in vacuo. [Wolfenden et al. J Am Chem Soc 109 463 1987, Beilstein 6 IV 5717.]
Incompatibilities
Strong oxidizers, strong bases, acid chlorides, acid anhydrides. Under certain conditions, a dust cloud can probably explode if ignited by a spark or flame.
Properties of 4-Methoxyphenol
| Melting point: | 56 °C |
| Boiling point: | 243 °C(lit.) |
| Density | 1,55 g/cm3 |
| vapor density | 4.3 (vs air) |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index | 1.5286 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in acetone, ethyl acetate, ethanol, ether, benzene and carbon tetrachloride. |
| form | Liquid |
| pka | 10.21(at 25℃) |
| color | Clear colorless to pale yellow |
| Odor | at 1.00?%?in?dipropylene glycol. phenolic |
| PH | 5.1 (30g/l, H2O, 20℃) |
| Odor Threshold | 0.0027ppm |
| Water Solubility | 40 g/L (25 ºC) |
| BRN | 507924 |
| Exposure limits | ACGIH: TWA 5 mg/m3 NIOSH: TWA 5 mg/m3 |
| Stability: | Stable. Combustible. Incompatible with halogens, oxidizing agents. |
| CAS DataBase Reference | 150-76-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Mequinol(150-76-5) |
| EPA Substance Registry System | p-Methoxyphenol (150-76-5) |
Safety information for 4-Methoxyphenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Methoxyphenol
| InChIKey | NWVVVBRKAWDGAB-UHFFFAOYSA-N |
4-Methoxyphenol manufacturer
Clickchem Research LLP
Krystal Tech
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 4- Methoxy phenol 98%View Details
4- Methoxy phenol 98%View Details -
 Hydroquinone monomethyl ether 98%View Details
Hydroquinone monomethyl ether 98%View Details -
 Hydroquinone monomethyl ether 98%View Details
Hydroquinone monomethyl ether 98%View Details -
 4-Methoxy phenol CAS 150-76-5View Details
4-Methoxy phenol CAS 150-76-5View Details
150-76-5 -
 Hydroquinone Monomethyl Ether CASView Details
Hydroquinone Monomethyl Ether CASView Details -
 Hydroquinone mono-methyl ether CAS 150-76-5View Details
Hydroquinone mono-methyl ether CAS 150-76-5View Details
150-76-5 -
 Hydroquinone monomethyl ether 99% CAS 150-76-5View Details
Hydroquinone monomethyl ether 99% CAS 150-76-5View Details
150-76-5 -
 4-MethoxyphenolView Details
4-MethoxyphenolView Details
150-76-5
