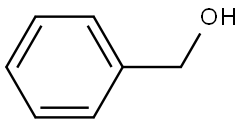
Benzene
Synonym(s):Benzene;Benzene in dimethyl sulfoxide;Benzene solution;Benzine;Benzol
- CAS NO.:71-43-2
- Empirical Formula: C6H6
- Molecular Weight: 78.11
- MDL number: MFCD00003009
- EINECS: 200-753-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
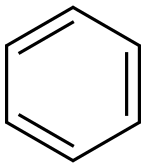
What is Benzene?
Description
Benzene (C6H6) is an aromatic hydrocarbon compound widely used in the chemical industry. Its very stable structure makes it less susceptible to addition reactions and often participates in substitution reactions, including alkylation, halogenation, and nitration. It is sometimes used as a solvent in certain reactions. Because benzene is classified as a human carcinogen, its use is generally avoided. Toluene is often a suitable substitute for benzene.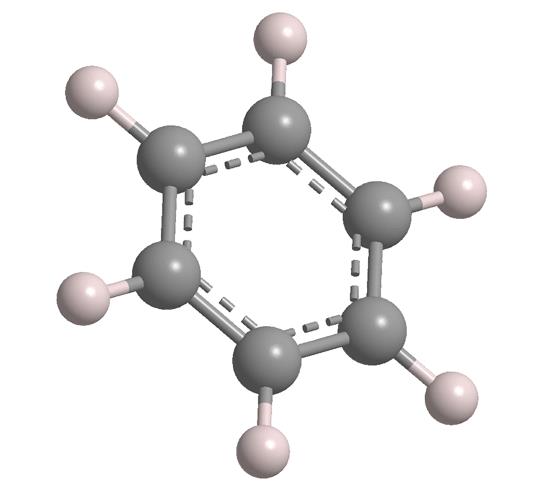
Chemical properties
Benzene is a clear, volatile, colorless, highly flammable liquid with a pleasant characteristic odor. Its boiling point is 80.1°C. The odor threshold concentration of benzene in air at 40°C is 190 μg/L; the average minimum detectable odor threshold concentrations in water at 60°C and in air at 40°C are 0.072 mg/L and 0.5 mg/L, respectively.
History
Benzene was discovered in 1825 by Michael Faraday (1791–1867), who identified it in a liquid residue from heated whale oil. Faraday called the compound bicarburet of hydrogen, and its name was later changed to benzin by Eilhardt Mitscherlich (1794–1863), who isolated the compound from benzoin (C14H12O2).
The Uses of Benzene
Benzene is soluble in alcohol, ether, chloroform and glacial acetic acid, but insoluble in water. It has a wide range of uses, including:
(1) Used in the manufacture of ethylbenzene, dodecylbenzene, cyclohexane, phenol, nitrobenzene, maleic anhydride, chlorobenzene, biphenyl, hexachlorobenzene, benzenesulfonic acid and other derivatives.
(2) Used as a solvent for wax, gum, resin and amber.
(3) Used as a diluent for varnish, used to modify the varnish on silver bromide gelatin negatives.
(4) Used in the production of nylon and synthetic fibers.
(5) Used in the manufacture of dyes, drugs, varnishes and linoleum.
Definition
ChEBI: Benzene is a six-carbon aromatic annulene in which each carbon atom donates one of its two 2p electrons into a delocalised pi system. A toxic, flammable liquid byproduct of coal distillation, it is used as an industrial solvent. Benzene is a carcinogen that also damages bone marrow and the central nervous system.
Production Methods
Today benzene, which is a natural component of petroleum, is obtained from petroleum by several processes. Toluene hydrodealkylation involves mixing toluene (C6H5CH3) and hydrogen in the presence of catalysts and temperatures of approximately 500°C and pressures of about 50 atmospheres to produce benzene and methane: C6H5CH3 + H2 → C6H6 + CH4. Hydrodealkylation strips the methyl group from toluene to produce benzene. Toluene disproportionation involves combining toluene so that the methyl groups bond to one aromatic ring, producing benzene and xylene. Benzene can also be obtained from petroleum reforming in which temperature, pressure, and catalysts are used to convert petroleum components to benzene, which can then be extracted using solvents and distillation processes. Another source of benzene is pyrolysis gasoline or pygas.
Reactions
Benzene reacts (1) with chlorine, to form (a) substitution products (one-half of the chlorine forms hydrogen chloride) such as chlorobenzene, C6H5Cl; dichlorobenzene, C6H4Cl2(1,4) and (1,2); trichlorobenzene, C6H3Cl3(1,2,4); tetrachlorobenzene (1,2,3,5); and (b) addition products, such as benzene dichloride C6H6Cl2; benzene tetrachloride, C6H6Cl4; and benzene hexachloride, C6H6Cl6. The formation of substitution products of the benzene nucleus, whether in benzene or its homologues, is favored by the presence of a catalyzer, e.g., iodine, phosphorus, iron; (2) with concentrated HNO3, to form nitrobenzene, C6H5NO2; 1,3- dinitrobenzene, C6H4(NO2)2 (1,3), 1,3,5-trinitrobenzene, C6H3(NO2)3 (1,3,5); (3) with concentrated H2SO4, to form benzene sulfonic acid, C6H5SO3H, benzene disulfonic acid, C6H4(SO3H)2(1,3), benzene trisulfonic acid, C6H3(SO3H)3 (1,3–5); (4) with methyl chloride plus anhydrous aluminum chloride (Friedel-Crafts reaction) to form toluene, monomethyl benzene, C6H5CH3; dimethyl benzene C6H4(CH3)2; trimethyl benzene, C6H3(CH3)3; (5) with acetyl chloride plus anhydrous aluminum chloride (Friedel-Crafts reaction) to form acetophenone (methylphenyl ketone), C6H5COCH3.
General Description
Benzene appears as a clear colorless liquid with a petroleum-like odor. Flash point less than 0 °F. Less dense than water and slightly soluble in water. Hence floats on water. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Benzene reacts vigorously with allyl chloride or other alkyl halides even at minus 70°C in the presence of ethyl aluminum dichloride or ethyl aluminum sesquichloride. Explosions have been reported [NFPA 491M 1991]. Ignites in contact with powdered chromic anhydride [Mellor 11:235 1946-47]. Incompatible with oxidizing agents such as nitric acid. Mixtures with bromine trifluoride, bromine pentafluoride, iodine pentafluoride, iodine heptafluoride and other interhalogens can ignite upon heating [Bretherick 5th ed. 1995]. Benzene and cyanogen halides yield HCl as a byproduct (Hagedorn, F. H. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.). The reaction of Benzene and trichloroacetonitrile evolves toxic chloroform and HCl gasses. (Hagedorn, F., H.-P. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).
Hazard
The acute toxicity of benzene is low. Inhalation of benzene can cause dizziness, euphoria, giddiness, headache, nausea, drowsiness, and weakness. Benzene can cause moderate irritation to skin and severe irritation to eyes and mucous membranes. Benzene readily penetrates the skin to cause the same toxic effects as inhalation or ingestion. The chronic toxicity of benzene is significant. Exposure to benzene affects the blood and blood-forming organs such as the bone marrow, causing irreversible injury; blood disorders including anemia and leukemia may result. The symptoms of chronic benzene exposure may include fatigue, nervousness, irritability, blurred vision, and labored breathing. Benzene is regulated by OSHA as a carcinogen (Standard 1910.1028) and is listed in IARC Group 1 ("carcinogenic to humans"). This substance is classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard.
Flammability and Explosibility
Benzene is a highly flammable liquid (NFPA rating = 3), and its vapors may travel a considerable distance to a source of ignition and "flash back." Vapor-air mixtures are explosive above the flash point. Carbon dioxide and dry chemical extinguishers should be used to fight benzene fires.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Industrial uses
Benzene (C6H6, CAS No. 71-43-2) is an aromatic hydrocarbon compound used extensively in the chemical industry as an intermediate in the manufacture of polymers and other products. It is also a common atmospheric contaminant and is present in motor vehicle exhaust emissions and cigarette smoke.
In 1990, it was discovered by the USA soft drinks industry that benzene could be produced at low levels in certain soft drinks containing a benzoate preservative and ascorbic acid. Since benzene is a known human carcinogen, its presence in food and beverages is clearly undesirable.
Biochem/physiol Actions
Environmental carcinogen; hematoxin that is linked to increased incidence of leukemia in humans.
Safety Profile
Confirmed human carcinogen producing myeloid leukemia, Hodgkin's dsease, and lymphomas by inhalation. Experimental carcinogenic, neoplastigenic, and tumorigenic data. A human poison by inhalation. An experimental poison by skin contact, intraperitoneal, intravenous, and possibly other routes. Moderately toxic by ingestion and subcutaneous routes. A severe eye and moderate sktn irritant. Human systemic effects by inhalation and ingestion: blood changes, increased body temperature. Experimental teratogenic and reproductive effects. Human mutation data reported. A narcotic. In industry, inhalation is the primary route of chronic benzene poisoning. Poisoning by skin contact has been reported. Recent (1 987) research indicates that effects are seen at less than 1 ppm. Exposures needed to be reduced to 0.1 ppm before no toxic effects were observed. Elimination is chiefly through the lungs.
Potential Exposure
Benzene is used as a constituent in motor fuels; as a solvent for fats; inks, oils, paints, plastics, and rubber, in the extraction of oils from seeds and nuts; in photogravure printing. It is also used as a chemical intermediate. By alkylation, chlorination, nitration, and sulfonation, chemicals, such as styrene, phenols, and malefic anhydride are produced. Benzene is also used in the manufacture of detergents, explosives, pharmaceuticals; in the manufacture of cyclohexane and ethylbenzene; and dye-stuffs. Increased concern for benzene as a significant environmental pollutant arises from public exposure to the presence of benzene in gasoline and the increased content in gasoline due to requirements for unleaded fuels for automobiles equipped with catalytic exhaust converters.
First aid
Eyes, skin, respiratory system, blood, central nervous system, bone marrow. Cancer site: leukemia.
Carcinogenicity
Benzene is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.
Environmental Fate
Benzene is released to air primarily by vaporization and combustion emissions associated with its use in gasoline. Other sources are vapors from its production and use in manufacturing other chemicals. In addition, benzene may be in industrial effluents discharged into water and accidental releases from gas and oil production, refining and distribution industries. Benzene released to soil will either evaporate very quickly or leach to groundwater. It can be biodegraded by soil and groundwater microbes. Benzene released to surface water should mostly evaporate within a few hours to a few days, depending on quantity, temperature, water turbulence, etc. Although benzene does not degrade by hydrolysis, it may be biodegraded by microbes.
Storage
work with benzene should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Benzene should be used only in areas free of ignition sources.
Shipping
UN1114 Benzene, Hazard Class: 3; Labels: 3— Flammable liquid
Purification Methods
For most purposes, *benzene can be purified sufficiently by shaking with conc H2SO4 until free from thiophene, then with H2O, dilute NaOH and water, followed by drying (with P2O5, sodium, LiAlH4, CaH2, 4X Linde molecular sieve, or CaSO4, or by passage through a column of silica gel, and for a preliminary drying, CaCl2 is suitable), and distillation. A further purification step to remove thiophene, acetic acid and propionic acid, is crystallisation by partial freezing. The usual contaminants in dry thiophene-free *benzene are non-benzenoid hydrocarbons such as cyclohexane, methylcyclohexane, and heptanes, together with naphthenic hydrocarbons and traces of toluene. Carbonyl-containing impurities can be removed by percolation through a Celite column impregnated with 2,4-dinitrophenylhydrazine, phosphoric acid and H2O. (Prepared by dissolving 0.5g DNPH in 6mL of 85% H3PO4 by grinding together, then adding and mixing 4mL of distilled H2O and 10g Celite.) [Schwartz & Parker Anal Chem 33 1396 1961.] *Benzene has been freed from thiophene by refluxing with 10% (w/v) of Raney nickel for 15minutes, after which the nickel is removed by filtration or centrifugation. Dry *benzene is obtained by doubly distilling high purity *benzene from a solution containing the blue ketyl formed by the reaction of sodium-potassium alloy with a small amount of benzophenone. Thiophene has been removed from *benzene (absence of bluish-green coloration when 3mL of *benzene is shaken with a solution of 10mg of isatin in 10mL of conc H2SO4) by refluxing the *benzene (1.25L) for several hours with 40g HgO (freshly precipitated) dissolved in 40mL glacial acetic acid and 300mL of water. The precipitate is filtered off, the aqueous phase is removed and the *benzene is washed twice with H2O, dried and distilled. Alternatively, *benzene dried with CaCl2 has been shaken vigorously for 0.5hour with anhydrous AlCl3 (12g/L) at 25-35o, then decanted, washed with 10% NaOH, and water, dried and distilled. The process is repeated, giving thiophene-free *benzene. [Holmes & Beeman Ind Eng Chem 26 172 1934.] After shaking successively for about an hour with conc H2SO4, distilled water (twice), 6M NaOH, and distilled water (twice), *benzene is distilled through a 3-ft glass column to remove most of the water. Absolute EtOH is added and the *benzene-alcohol azeotrope is distilled. (This low-boiling distillation leaves any non-azeotrope-forming impurities behind.) The middle fraction is shaken with distilled water to remove EtOH, and again redistilled. Final slow and very careful fractional distillation from sodium, then LiAlH4 under N2, removed traces of water and peroxides. [Peebles et al. J Am Chem Soc 82 2780 1960.] *Benzene liquid and vapour are very TOXIC and HIGHLY FLAMMABLE, and all operations should be carried out in an efficient fume cupboard and in the absence of naked flames in the vicinity. [Beilstein 5 H 175, 5 I 95, 5 II 119, 5 III 469.] Rapid purification: To dry benzene, alumina, CaH2 or 4A molecular sieves (3% w/v) may be used (dry for 6hours). Then benzene is distilled, discarding the first 5% of distillate, and stored over molecular sieves (3A, 4A) or Na wire.
Toxicity evaluation
Benzene enters the air, water, and soil as a result of industrial processes, emissions from burning coal and oil, tobacco smoke, gasoline exhaust, and gasoline leaks, and from natural sources, including volcanoes and forest fires. Benzene in the atmosphere chemically degrades in only a few days. Benzene released to soil or waterways is subject to volatilization, photooxidation, and biodegradation. Benzene has a short halflife in surface water because it is so volatile.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, many fluorides and perchlorates, nitric acid.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Dilution with alcohol or acetone to minimize smoke is recommended. Bacterial degradation is also possible.
Regulations
Current USA and EU legislation does not set maximum limits for benzene in soft drinks. However, the FDA has adopted the Environmental Protection Agency (EPA) maximum contaminant level (MCL) for drinking water of 5 ppb as a quality standard for bottled water. ThisMCL has been used to evaluate the significance of benzene contamination in the soft drinks tested in surveys. The FSA has used the World Health Organization (WHO) guideline level for benzene in water of 10 mg kg-1 as a point of reference for its own survey results.
Properties of Benzene
| Melting point: | 5.5 °C (lit.) |
| Boiling point: | 80 °C (lit.) |
| Density | 0.874 g/mL at 25 °C (lit.) |
| vapor density | 2.77 (vs air) |
| vapor pressure | 166 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | 12 °F |
| storage temp. | room temp |
| solubility | Miscible with alcohol, chloroform, dichloromethane, diethyl ether, acetone and acetic acid. |
| form | Liquid |
| appearance | colorless liquid |
| pka | 43(at 25℃) |
| color | APHA: ≤10 |
| Odor | Paint-thinner-like odor detectable at 12 ppm |
| Relative polarity | 0.111 |
| explosive limit | 1.4-8.0%(V) |
| Odor Threshold | 2.7ppm |
| Water Solubility | 0.18 g/100 mL |
| λmax | λ: 280 nm Amax: 1.0 λ: 290 nm Amax: 0.15 λ: 300 nm Amax: 0.06 λ: 330 nm Amax: 0.02 λ: 350-400 nm Amax: 0.01 |
| Merck | 14,1066 |
| BRN | 969212 |
| Henry's Law Constant | 10.4 at 45.00 °C, 11.4 at 50.00 °C, 13.3 at 55.00 °C, 14.5 at 60.00 °C, 16.8 at 65.00 °C, 19.2 at
70.00 °C (static headspace-GC, Park et al., 2004) |
| Exposure limits | TLV-TWA 10 ppm (~32 mg/m3) (ACGIH
and OSHA); ceiling 25 ppm (~80 mg/m3)
(OSHA and MSHA); peak 50 ppm (~160
mg/m3)/10 min/8 h (OSHA); carcinogenicity:
Suspected Human Carcinogen (ACGIH),
Human Sufficient Evidence (IARC). |
| Dielectric constant | 2.3(20℃) |
| Stability: | Stable. Substances to be avoided include strong oxidizing agents, sulfuric acid, nitric acid, halogens. Highly flammable. |
| CAS DataBase Reference | 71-43-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene(71-43-2) |
| IARC | 1 (Vol. 29, Sup 7. 100F, 120) 2018 |
| EPA Substance Registry System | Benzene (71-43-2) |
Safety information for Benzene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H340:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Benzene
| InChIKey | UHOVQNZJYSORNB-UHFFFAOYSA-N |
Benzene manufacturer
Sona Enterprise
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Benzene 98%View Details
Benzene 98%View Details -
 Benzene 99%View Details
Benzene 99%View Details -
 Benzene 99%View Details
Benzene 99%View Details -
 Benzene CASView Details
Benzene CASView Details -
 Benzene Liquid ChemicalView Details
Benzene Liquid ChemicalView Details
71-43-2 -
 Liquid Benzene ChemicalView Details
Liquid Benzene ChemicalView Details
71-43-2 -
 BENZENE 99.5% ARView Details
BENZENE 99.5% ARView Details
71-43-2 -
 Liquid Benzene Chemical, Grade Standard: Reagent Grade, for LaboratoryView Details
Liquid Benzene Chemical, Grade Standard: Reagent Grade, for LaboratoryView Details
71-43-2