Tamoxifen
Synonym(s):(Z)-1-(p-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene;trans-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine;Tamoxifen
- CAS NO.:10540-29-1
- Empirical Formula: C26H29NO
- Molecular Weight: 371.51
- MDL number: MFCD00010454
- EINECS: 234-118-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
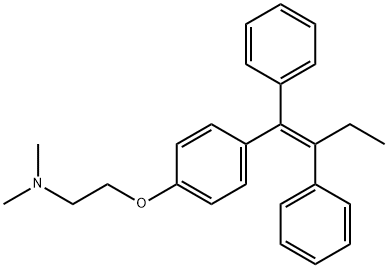
What is Tamoxifen?
Absorption
An oral dose of 20mg reaches a Cmax of 40ng/mL with a Tmax of 5 hours. The metabolite N-desmethyltamoxifen reaches a Cmax of 15ng/mL. 10mg of tamoxifen orally twice daily for 3 months results in a Css of 120ng/mL and a Css of 336ng/mL.
Toxicity
High doses of tamoxifen in animals lead to respiratory difficulty and convulsions. High doses in advanced metastatic cancer patients resulted in acute neurotoxicity seen by tremor, hyperreflexia, unsteady gait, and dizziness. Patients experiencing and overdose should be given supportive treatment as no specific treatment for overdose is suggested.
Description
In 1966, ICI Pharmaceuticals (now AstraZeneca) first synthesized tamoxifen in the hope of developing a morning-after contraceptive pill. The UK patent for this compound was in place in 1962, whereas the US patent was repeatedly denied until the 1980s. Tamoxifen was approved for a fertility treatment but it was not proven as useful in regulating human contraception. Even though there was a link between estrogen and breast cancer, developing a cancer treatment was not a priority at the time. In 1971, the first clinical study showed a convincing effect of tamoxifen in treating advanced breast cancer. From 1971 to 1977, this drug was neither clinically nor financially remarkable. In 1980s, however, publications first showed that tamoxifen, in addition to chemotherapy, improved survival for patients with early stage breast cancer. In 1998, the meta-analysis by the Oxford-based Early Breast Cancer Trialists’ Collaborative Group showed that tamoxifen did indeed save lives in early breast cancer. In 2001, tamoxifen sales were over $1.024 billion. Since the expiration of the patent in 2002, it is now widely available as a generic drug. By 2004, tamoxifen was the best selling hormonal drug for the treatment of breast cancer.
Description
Tamoxifen has been the key drug for treating breast cancer for decades. It selectively blocks the estrogen receptor, making it effective in treating diseases, such as breast cancer, where cell behavior is controlled by estrogen.
Chemical properties
White Crystalline Solid
Originator
Nolvadex,I.C.I.,UK,1973
The Uses of Tamoxifen
A nonsteroidal estrogen antagonist of interest in the treatment of some forms of breast cancer. Tamoxifen is a Protein Kinase C inhibitor, and induces apoptosis in human malignant glioma cell lines
The Uses of Tamoxifen
Tamoxifen is a selective estrogen response modifier (SERM), protein kinase C inhibitor and anti-angiogenetic factor. Tamoxifen is a prodrug that is metabolized to active metabolites 4-hydroxytamoxifen (4-OHT) and endoxifen by cytochrome P450 isoforms CYP2D6 and CYP3A4. In breast cancer, the gene repressor activity of tamoxifen against ERBB2 is dependent upon PAX2. Blocks estradiol-stimulated VEGF production in breast tumor cells. Protein kinase C inhibitor. Induces apoptosis in human malignant glioma cell lines. Tamoxifen and its metabolite 4-hydroxytamoxifen are selective estrogen response mo difiers (SERMs) that act as estrogen antagonists in mammary gland. Blocks estradiol-stimulated VEGF production in breast tumor cells.
The Uses of Tamoxifen
Tamoxifen has been used to facilitate the recombination of ect2flox allele in mouse organs91. It has also been used to study its effect on lipopolysaccharide (LPS)-induced microglial activation92.
Background
Tamoxifen is a non-steroidal antiestrogen used to treat estrogen receptor positive breast cancers as well as prevent the incidence of breast cancer in high risk populations. Tamoxifen is used alone or as an adjuvant in these treatments. Tamoxifen may no longer be the preferred treatment for these types of cancers as patients generally have better survival, side effect profiles, and compliance with anastrozole.
Tamoxifen was granted FDA approval on 30 December 1977.
Indications
Tamoxifen is indicated to treat estrogen receptor positive metastatic breast cancer in adults, as an adjuvant in the treatment of early stage estrogen receptor positive breast cancer in adults, to reduce the risk of invasive breast cancer after surgery and radiation in adult women with ductal carcinoma in situ.
What are the applications of Application
Tamoxifen is a selective estrogen response modifier (SERM), protein kinase C inhibitor and anti-angiogenetic factor
Indications
Tamoxifen (Nolvadex) is a synthetic antiestrogen used in the treatment of breast cancer. Normally, estrogens act by binding to a cytoplasmic protein receptor, and the resulting hormone–receptor complex is then translocated into the nucleus, where it induces the synthesis of ribosomal RNA (rRNA) and messenger RNA (mRNA) at specific sites on the DNA of the target cell. Tamoxifen also avidly binds to estrogen receptors and competes with endogenous estrogens for these critical sites. The drug–receptor complex has little or no estrogen agonist activity.Tamoxifen directly inhibits growth of human breast cancer cells that contain estrogen receptors but has little effect on cells without such receptors.
Indications
Tamoxifen is a partial estrogen agonist in breast and thus is used as a treatment and chemopreventative for breast cancer. Tamoxifen is a full agonist in bone and endometrium, and prolonged use of tamoxifen leads to a fourfold to fivefold increase in the incidence of endometrial cancer. See Chapter 56 for a detailed discussion of the use of tamoxifen in breast cancer.
Definition
ChEBI: Tamoxifen is a tertiary amino compound and a stilbenoid. It has a role as an estrogen receptor antagonist, a bone density conservation agent, an estrogen receptor modulator, an estrogen antagonist, an angiogenesis inhibitor, an EC 2.7.11.13 (protein kinase C) inhibitor, an EC 1.2.3.1 (aldehyde oxidase) inhibitor and an antineoplastic agent. It derives from a hydride of a stilbene.
Manufacturing Process
To the Grignard reagent prepared from 0.59 part of magnesium, 3.95 parts of
bromobenzene and 50 parts of ether there are added 7.5 parts of 4-(β-
dimethylaminoethoxy)-α-ethyldesoxybenzoin in 50 parts of ether. After
heating under reflux for 3 hours, the mixture is decomposed by the addition
of a solution of 60 parts of ammonium chloride in 150 parts of water. The
mixture is separated, and the ethereal layer is dried with anhydrous sodium
sulfate, and the ether is evaporated. The residue is crystallized from
methanol. There is thus obtained 1-(p-β-dimethylaminoethoxyphenyl)-1,2-
diphenylbutan-1-ol, melting point 120°C to 121°C.
2.15 parts of 1-(p-β-dimethylaminoethoxyphenyl)-1,2-diphenylbutan-1-ol, 25
parts of ethanol and 0.8 part of 10 N hydrochloric acid are heated together
under reflux for 3 hours. The solution is evaporated to dryness under reduced
pressure and the residue is extracted with methylene chloride. The methylene
chloride extract is decolorized with charcoal and then evaporated to dryness.
The residue is dissolved in 100 parts of water, the solution is basified by the
addition of sodium hydroxide solution, and the precipitated solid is extracted
three times, each time with 50 parts of ether. The combined extracts are dried with anhydrous sodium sulfate and then evaporated. The residue is
crystallized from aqueous methanol, and there is thus obtained 1-(p-β-
dimethylaminoethoxyphenyl)-1,2-diphenylbut-1-ene, melting point 95°C to
96°C.
brand name
Nolvadex (AstraZeneca); Soltamox (Savient).
Therapeutic Function
Antiestrogen, Antineoplastic
World Health Organization (WHO)
Tamoxifen is an anti-estrogen agent used mainly to treat breast cancer. Tamoxifen is listed in the WHO Model List of Essential Drugs.
General Description
Tamoxifen is a selective estrogen response modifier (SERM), protein kinase C inhibitor and anti-angiogenetic factor. Tamoxifen is a prodrug that is metabolized to active metabolites 4-hydroxytamoxifen (4-OHT) and endoxifen by cytochrome P450 isoforms CYP2D6 and CYP3A4. In breast cancer, the gene repressor activity of tamoxifen against ERBB2 is dependent upon PAX2. Blocks estradiol-stimulated VEGF production in breast tumor cells.
Biochem/physiol Actions
Protein kinase C inhibitor. Induces apoptosis in human malignant glioma cell lines. Tamoxifen and its metabolite 4-hydroxytamoxifen are selective estrogen response modifiers (SERMs) that act as estrogen antagonists in mammary gland. Blocks estradiol-stimulated VEGF production in breast tumor cells.
Mechanism of action
Tamoxifen is slowly absorbed, and maximum serum levels are achieved 4 to 7 hours after oral administration. The drug is concentrated in estrogen target tissues, such as the ovaries, uterus, vaginal epithelium, and breasts. Hydroxylation and glucuronidation of the aromatic rings are the major pathways of metabolism; excretion occurs primarily in the feces.
Pharmacokinetics
Tamoxifen is a selective estrogen receptor modulator that inhibits growth and promotes apoptosis in estrogen receptor positive tumors. It has a long duration of action as the active metabolite N-desmethyltamoxifen has a half life of approximately 2 weeks. It has a narrow therapeutic index as higher doses can lead to breathing difficulty or convulsions. Tamoxifen administration is also associated with an increased incidence of uterine malignancies.
Pharmacokinetics
Circulating levels of the demethylated metabolite at steady state are up to twice the level of the parent drug, because the elimination half-life of N-demethyl tamoxifen is 14 days, compared with 7 days for tamoxifen. Tamoxifen demonstrates only weak estrogenic effects at several sites, including the endometrium and bone, and on the lipid profile. Tamoxifen undergoes rapid N-dem ethylation to its major metabolite, N-dem ethyltamoxifen, by CYP3A4 and via CYP2D6 to its minor metabolite, 4-hydroxytam oxifen. Evidence suggests that 4-hydroxytamoxifen is the active metabolite of tamoxifen, with a higher binding affinity than the parent drug for the ER
Clinical Use
Tamoxifen is a SERM that is used as an antiestrogen in the treatment of estrogen-dependent breas Tcancer following prim ary treatment (c hemotherapy and/or surgery).
Side Effects
Tamoxifen administration is associated with few toxic side effects, most frequently hot flashes (in 10–20% of patients) and occasionally vaginal dryness or discharge. Mild nausea, exacerbation of bone pain, and hypercalcemia may occur.
Safety Profile
Confirmed human carcinogen. Moderately toxic by ingestion and intraperitoneal routes. Human systemic effects by an unspecified route: nausea or vomiting, leukopenia, thrombocytopenia, and skin changes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
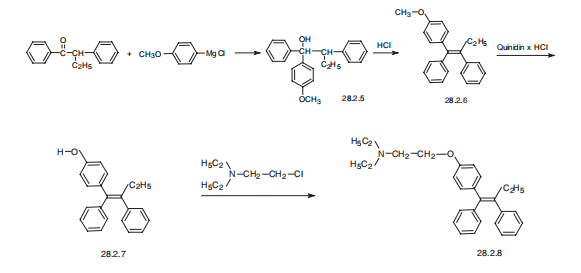
Synthesis
Tamoxifen, (Z)-2-[p-(1,2-diphenyl-1-butenyl)phenoxy]N,N-dimethylethylamine (28.2.8), is synthesized from |á-ethyldezoxybenzoin. Interaction of this with 4- methoxyphenylmagnesium bromide gives the corresponding carbinol (28.2.5). Its dehydration in acidic conditions gives a derivative of stilbene (28.2.6), and further heating of which with quinidine hydrochloride as a demethylating agent gives 2-[p-(1,2-diphenyl- 1-butenyl)phenol] (28.2.7). The phenolic hydroxyl is further alkylated by dimethylaminoethylchoride using sodium ethoxide as a base, which forms a mixture of E and Z isomers of the final product. The desired Z isomer, tamoxifen (28.2.8) is isolated by fractional crystallization from petroleum ester.
in vitro
ic50s for growth inhibition ranged from 5.5–10 μm, and were not affected by estrogen. tamoxifen-mediated growth inhibition was not associated with induction of tgf-β. however, tamoxifen treatment was associated with inhibition of pkc, which was followed by induction of p21waf1/cip1, rb dephosphorylation, and g1/s phase cell cycle arrest [1].
in vivo
the tumor cell kinetics of mcf-7 human breast carcinoma xenografts grown in nude mice can be significantly altered by hormonal manipu lation. tamoxifen treatment or e2 deprivation resulted in an approximate doubling of the tpol and an approximately 40% reduction in labeling index as compared to e2-stimulated tumors. an increase in cell loss rate was calculated for both tamoxifen treatment and e2 deprivation [2].
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins enhanced.
Antidepressants: metabolism of tamoxifen to active
metabolite possibly inhibited by fluoxetine and
paroxetine - avoid.
Antipsychotics: increased risk of ventricular
arrhythmias with droperidol - avoid.
Buproprion: metabolism of tamoxifen to active
metabolite possibly inhibited - avoid.
Cinacalcet: metabolism of tamoxifen to active
metabolite possibly inhibited - avoid.
Carcinogenicity
Tamoxifen is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.
Metabolism
Tamoxifen can by hydroxylated to α-hydroxytamoxifen which is then glucuronidated or undergoes sulfate conjugation by sulfotransferase 2A1. Tamoxifen can also undergo N-oxidation by flavin monooxygenases 1 and 3 to tamoxifen N-oxide. Tamoxifen is N-dealkylated to N-desmethyltamoxifen by CYP2D6, CYP1A1, CYP1A2, CYP3A4, CYP1B1, CYP2C9, CYP2C19, and CYP3A5. N-desmethyltamoxifen can be sulfate conjugated to form N-desmethyltamoxifen sulfate, 4-hydroxylated by CYP2D6 to form endoxifen, or N-dealkylated again by CYP3A4 and CYP3A5 to N,N-didesmethyltamoxifen. N,N-didesmethyltamoxifen undergoes a substitution reaction to form tamoxifen metabolite Y, followed by ether cleavage to metabolite E, which can then be sulfate conjugated by sulfotransferase 1A1 and 1E1 or O-glucuronidated.
Tamoxifen can also by 4-hydroxylated by CYP2D6, CYP2B6, CYP3A4, CYP2C9, and CYP2C19 to form 4-hydroxytamoxifen. 4-hydroxytamoxifen can undergo glucuronidation by UGT1A8, UGT1A10, UGT2B7, and UGT2B17 to tamoxifen glucuronides, sulfate conjugation by sulfotransferase 1A1 and 1E1 to 4-hydroxytamoxifen sulfate, or N-dealkylation by CYP3A4 and CYP3A5 to endoxifen.
Endoxifen undergoes demethylation to norendoxifen, a reversible sulfate conjugation reaction via sulfotransferase 1A1 and 1E1 to 4-hydroxytamoxifen sulfate, sulfate conjugation via sulfotransferase 2A1 to 4-endoxifen sulfate, or glucuronidation via UGT1A8, UGT1A10, UGT2B7, or UGT2B15 to tamoxifen glucuronides.
Metabolism
Tamoxifen is extensively metabolised by cytochrome
P450 isoenzymes, to active metabolites that include
N-desmethyltamoxifen, 4-hydroxytamoxifen, and
4-hydroxy-N-desmethyltamoxifen (endoxifen).
Metabolism is by hydroxylation, demethylation and
conjugation.
In-vitro studies suggest that both N-desmethyltamoxifen
and 4-hydroxytamoxifen are further metabolised to
endoxifen.
Elimination occurs, chiefly as conjugates with practically
no unchanged drug, principally through the faeces and to
a lesser extent through the kidneys.
storage
Room temperature
References
[1] rohlff c, blagosklonny mv, kyle e, kesari a, kim iy, zelner dj, hakim f, trepel j, bergan rc. prostate cancer cell growth inhibition by tamoxifen is associated with inhibition of protein kinase c and induction of p21(waf1/cip1). prostate. 1998 sep 15;37(1):51-9.
[2] jann n. sarkaria, david f. c. gibson, v. craig jordan, john f. fowler, mary j. lindstrom, andr. timothy mulcahy. tamoxifen-induced increase in the potential doubling time of mcf-7 xenografts as determined by bromodeoxyuridine labeling and flow cytometry. cancer research 5.1. 4413-1417, september 15, 1993.
[3] osborne ck. tamoxifen in the treatment of breast cancer. n engl j med. 1998 nov 26;339(22):1609-18.
Properties of Tamoxifen
| Melting point: | 97-98 °C(lit.) |
| Boiling point: | 501.18°C (rough estimate) |
| Density | 1.0630 (rough estimate) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: insoluble <0.1% at 20°C |
| form | neat |
| pka | pKa 8.71(H2O
t = 25
I = 0.025) (Uncertain) |
| form | Solid |
| color | Crystals from pet ether |
| Water Solubility | Insoluble in water. Soluble in methanol, ethanol, propanol or propylene glycol.Soluble in dimethyl sulfoxide, dichloromethane and ethanol. Insoluble in water. |
| Merck | 13,9137 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 10540-29-1(CAS DataBase Reference) |
| IARC | 1 (Vol. 66, 100A) 2012 |
| EPA Substance Registry System | Tamoxifen (10540-29-1) |
Safety information for Tamoxifen
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H350:Carcinogenicity H360:Reproductive toxicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Tamoxifen
| InChIKey | NKANXQFJJICGDU-QPLCGJKRSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Tamoxifen 98% CAS 10540-29-1View Details
Tamoxifen 98% CAS 10540-29-1View Details
10540-29-1 -
 Tamoxifen CAS 10540-29-1View Details
Tamoxifen CAS 10540-29-1View Details
10540-29-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1