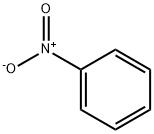
1,2-Dinitrobenzene
- CAS NO.:528-29-0
- Empirical Formula: C6H4N2O4
- Molecular Weight: 168.11
- MDL number: MFCD00007093
- EINECS: 208-431-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
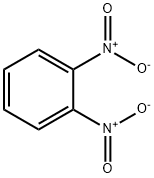
What is 1,2-Dinitrobenzene?
Description
Dinitrobenzene exists in three isomers (o-, m-,and p-); the meta form is the most widely used. All arewhite to yellow crystalline solids having a characteristicodor. Molecular weight= 168.12; Boiling point = (o-)318℃; (m-) 300℃; (p-) 299℃; (mixed) 305℃; Freezing/Melting point = (o-) 117118℃; (m-) 90℃; (p-)173174℃; (mixed) 7585℃; Flash point = (o-, m-,and p-) 149150℃. NFPA 704 M Hazard Identification(ortho-): Health 3, Flammability 1, Reactivity 4. Solubilityin water is poor for o-, m-, and mixed isomers; none forp-isomer.
Chemical properties
light yellow-brown powder
The Uses of 1,2-Dinitrobenzene
1,2-Dinitrobenzene was used as internal standard for analysis of the explosives, TNT, RDX, and tetryl in sea water by vapor phase chromatography with the nickel-63 electron capture detector.
Definition
ChEBI: 1,2-dinitrobenzene is a dinitrobenzene.
Synthesis Reference(s)
The Journal of Organic Chemistry, 21, p. 1065, 1956 DOI: 10.1021/jo01116a003
General Description
Colorless to yellow solid. Sinks and slowly mixes with water.
Air & Water Reactions
Slowly mixes with water.
Reactivity Profile
All three isomers have similar properties and may react vigorously with oxidizing materials. Their reaction with nitric acid (nitration) will lead to a mixture of trinitrobenzenes possessing high-explosive properties [Urbanski, 1967, vol. 3, p. 290]. If heat and reaction conditions of the nitration are not controlled, detonation comparable to TNT may occur [Anon., J. R. Inst. Chem., 1960, 84, p. 451]. Mixture of 1,3-dinitrobenzene with tetranitromethane was found highly explosive [Urbanski, 1964, vol. 1, 592]. 1,2-dinitrobenzene is a severe explosion hazard when shocked or exposed to heat or flame. When heated to decomposition all dinitrobenzens emit toxic fumes of nitrogen oxides [Sax, 9th ed., 1996, p. 1374].
Health Hazard
INHALATION, INGESTION, OR SKIN ABSORPTION: Headache, vertigo and vomiting followed by exhaustion, numbness of the legs, staggering and collapse. Intense methemoglobinenia may lead to asphyxia severe enough to injure the CNS. EYES: Irritation. SKIN: Stains skin yellow.
Safety Profile
Suspected carcinogen. Poison by inhalation and ingestion. Moderately toxic by sktn contact. Can cause liver, kidney, and central nervous system injury. Combustible when exposed to heat or flame; can react vigorously with oxidzing materials. A severe explosion hazard when shocked or exposed to heat or flame. It is used in bursting charges and to fiu artillery shells. Mixtures with nitric acid are highly explosive. To fight fire, use water, Co2, dry chemical. Dangerous; when heated to decomposition it emits highly toxic fumes of NO, and explodes. See also mand pDINITROBENZENE and NITRO COMPOUNDS of AROMATIC HYDROCARBONS.
Potential Exposure
Compound Description (m-isomer):Mutagen; Reproductive Effector; Human Data; PrimaryIrritant; (o- and p-isomers) Mutagen. Dinitrobenzenes areused in the synthesis of dyestuffs, dyestuff intermediates,and explosives; in celluloid production.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Effects may be delayed; medical observation isrecommended.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine.
Environmental Fate
Biological. Under anaerobic and aerobic conditions using a sewage inoculum, 1,2-dinitrobenzene degraded to nitroaniline (Hallas and Alexander, 1983).
Photolytic. Low et al. (1991) reported that the nitro-containing compounds (e.g., 2,4-
dinitrophenol) undergo degradation by UV light in the presence of titanium dioxide yielding
ammonium, carbonate, and nitrate ions. By analogy, 1,2-dinitrobenzene should degrade forming
identical ions.
Chemical/Physical. Releases toxic nitrogen oxides when heated to decomposition (Sax and
Lewis, 1987). 1,2-Dinitrobenzene will not hydrolyze in water (Kollig, 1993).
Storage
(1) Color Code—Yellow Stripe: Reactivity Hazard;Store separately in an area isolated from flammables, combustibles, or other yellow-coded materials. (2) Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with dinitrobenzene you should betrained on its proper handling and storage. Dinitrobenzenemust be stored to avoid contact with strong oxidizers (suchas chloride, bromine, chlorine dioxide, nitrates, and permanganates), since violent reactions occur. Contact with causticsand chemically active metals (such as tin and zinc) mayevolve heat, causing a buildup in pressure. Store in tightlyclosed containers in a cool, well-ventilated area away fromshock or heat, which may cause this chemical to explode.Storage outdoors or in explosion-proof areas is preferred.Sources of ignition, such as smoking and open flames, areprohibited where dinitrobenzene is handled, used, or stored.Metal containers used in the transfer of 5 gallons or more ofdinitrobenzene should be grounded and bonded. Drums mustbe equipped with self-closing valves, pressure vacuum bungs,and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers ofdinitrobenzene. Wherever dinitrobenzene is used, handled,manufactured, or stored, use explosion-proof electrical equipment and fittings
Shipping
Dinitrobenzenes all require a “POISONOUS/TOXIC MATERIALS” label. They fall in Hazard Class 6.1and Packing Group II.
Purification Methods
Crystallise it from EtOH. [Beilstein 5 IV 738.]
Properties of 1,2-Dinitrobenzene
| Melting point: | 116 °C |
| Boiling point: | 319 °C773 mm Hg(lit.) |
| Density | 1.57 |
| refractive index | 1.5650 |
| Flash point: | 150 °C |
| solubility | chloroform: soluble5%, clear, yellow-green |
| form | neat |
| color | Colorless to yellow needles |
| Water Solubility | 150 mg/L (20 ºC) |
| Merck | 14,3273 |
| BRN | 642224 |
| Exposure limits | NIOSH REL: TWA 1, IDLH 50; OSHA PEL: TWA 1; ACGIH TLV:
TWA 0.15 ppm for all isomers (adopted). |
| CAS DataBase Reference | 528-29-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1,2-dinitro-(528-29-0) |
| EPA Substance Registry System | o-Dinitrobenzene (528-29-0) |
Safety information for 1,2-Dinitrobenzene
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 1,2-Dinitrobenzene
1,2-Dinitrobenzene manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 1,2-Dinitrobenzene, 97% CAS 528-29-0View Details
1,2-Dinitrobenzene, 97% CAS 528-29-0View Details
528-29-0 -
 1,2-Dinitrobenzene CAS 528-29-0View Details
1,2-Dinitrobenzene CAS 528-29-0View Details
528-29-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
