2-Nitrochlorobenzene
- CAS NO.:88-73-3
- Empirical Formula: C6H4ClNO2
- Molecular Weight: 157.55
- MDL number: MFCD00007061
- EINECS: 201-854-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is 2-Nitrochlorobenzene?
Chemical properties
yellow solid with a characteristic odour
The Uses of 2-Nitrochlorobenzene
Intermediate, especially for dyes.
The Uses of 2-Nitrochlorobenzene
Chemical intermediate in manufacture of dyes, picric acid, lumber preservatives, and diaminophenol hydrochloride (a photographic developer)
The Uses of 2-Nitrochlorobenzene
1-Chloro-2-nitrobenzene was used in the synthesis of 1-hydroxybenzotriazole derivatives. It is important as a precursor to other compounds due to the two reactive sites present on the molecule.
Definition
ChEBI: 1-chloro-2-nitrobenzene is a C-nitro compound that is nitrobenzene in which one of the ortho- hydrogens has been replaced by chlorine. It is a C-nitro compound and a member of monochlorobenzenes.
Production Methods
Nitration of chlorobenzene with mixed acid (30/56/14) typically gives an isomer mix in 98 % yield consisting of 34 – 36 % 2-Nitrochlorobenzene, 63 – 65 % 4- chloronitrobenzene, and only ca. 1 % 3- chloronitrobenzene. The ortho – para ratio of about 0.55 is in sharp contrast to the 1.6 obtained with nitrotoluene isomers. As with the nitration of toluene, much work has been done on isomer control so that the producer might have some flexibility towards the balance of isomer demand. Although no major change in ratio has been achieved, the situation is better for nitrochlorobenzene than nitrotoluenes, because the favored isomer is in much greater demand. In further contrast to the nitration of toluene, the nitration of chlorobenzene in the presence of phosphoric acid decreases the proportion of 4-Nitrochlorobenzene, and the use of phosphoric acid in the presence of a transition-metal catalyst is said to increase the ortho – para ratio to ca. 0.8.
General Description
Yellow crystals with an aromatic odor. Sinks in water.
Air & Water Reactions
2-Nitrochlorobenzene may be sensitive to prolonged exposure to air and light. Insoluble in water.
Reactivity Profile
O-NITROCHLOROBENZENE is incompatible with strong bases and strong oxidizing agents. 2-Nitrochlorobenzene will react with ammonia.
Hazard
Toxic by inhalation and ingestion. Combustible. Questionable carcinogen.
Health Hazard
INHALATION: Headache, languour, anemia. Irritation of nose and throat, cyanosis, shallow respiration, convulsions, and coma. EYES: Irritation. SKIN: Irritation. INGESTION: Forms methemoglobin giving rise to cyanosis and blood changes.
Flammability and Explosibility
Not classified
Safety Profile
Suspected carcinogen with experimental carcinogenic and neoplastigenic data. Poison by ingestion, skin contact, and probably inhalation. Combustible when exposed to heat or flame. To fight fire, use water, foam. Potentially explosive reaction with ammonia at 160℃/30 bar. When heated to decomposition it emits toxic fumes of Cl-, NOx, and phosgene. See also other chloronitrobenzene entries and NITRO COMPOUNDS of AROMATIC HYDROCARBONS.
Purification Methods
Crystallise it from EtOH, MeOH or pentane (charcoal). [Beilstein 5 IV 721.]
Properties of 2-Nitrochlorobenzene
| Melting point: | 31-33 °C (lit.) |
| Boiling point: | 246 °C (lit.) |
| Density | 1.348 g/mL at 25 °C (lit.) |
| vapor density | 5.4 (vs air) |
| vapor pressure | 0.04 mm Hg ( 25 °C) |
| refractive index | 1.5520 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: soluble |
| form | Fused Crystalline Mass |
| color | Yellow |
| PH | 6 (0.4g/l, H2O, 20℃) |
| explosive limit | 1.15-13.1%(V) |
| Water Solubility | 0.43 g/L (20 ºC) |
| Merck | 14,2151 |
| BRN | 509273 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, reducing agents, strong bases, alkali metals. |
| CAS DataBase Reference | 88-73-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-chloro-2-nitro-(88-73-3) |
| IARC | 2B (Vol. 65, 123) 2020 |
| EPA Substance Registry System | o-Chloronitrobenzene (88-73-3) |
Safety information for 2-Nitrochlorobenzene
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 2-Nitrochlorobenzene
| InChIKey | BFCFYVKQTRLZHA-UHFFFAOYSA-N |
2-Nitrochlorobenzene manufacturer
Anand Agencies
Hemani Global
ASM Organics
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine N-Boc-2-bromoethylamine 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one N-octanoyl benzotriazole ELECTROLYTIC IRON POWDER 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 1-(2,4-DICHLOROPHENYL) ETHANAMINE 3-NITRO-2-METHYL ANILINE 9-methyl-9-azabicyclo [3.3.1] nonan-3-amine 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride 4-Bromopyrazole 1-Boc-4-cyanopiperidine 5-BROMO-2CYANO PYRIDINE (R)-5-(2-aminopropyl)-2-methoxybenzenesulfonamide 5,6-Dimethoxyindanone Tert-butyl N-(pent-4-yn-2-yl) carbamate TETRABUTYLAMMONIUM CYANIDE Cyclopropanamine hydrochloride 4-methoxybenzylbromide 3-Iodo-6-nitroindazoleRelated products of tetrahydrofuran
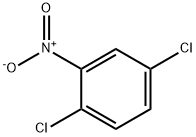

You may like
-
 o-Chloronitro benzene CAS 88-73-3View Details
o-Chloronitro benzene CAS 88-73-3View Details
88-73-3 -
 o-Nitrochlorobenzene CAS 88-73-3View Details
o-Nitrochlorobenzene CAS 88-73-3View Details
88-73-3 -
 1-CHLORO-2 NITROBENZENE For Synthesis CAS 88-73-3View Details
1-CHLORO-2 NITROBENZENE For Synthesis CAS 88-73-3View Details
88-73-3 -
 o-Nitrochlorobenzene (ONCB) pure CAS 88-73-3View Details
o-Nitrochlorobenzene (ONCB) pure CAS 88-73-3View Details
88-73-3 -
 1-Chloro-2-nitrobenzene CAS 88-73-3View Details
1-Chloro-2-nitrobenzene CAS 88-73-3View Details
88-73-3 -
 52-52-8 Cycloleucine 98+View Details
52-52-8 Cycloleucine 98+View Details
52-52-8 -
 1-Aminocyclobutanecarboxylic acid 98+View Details
1-Aminocyclobutanecarboxylic acid 98+View Details
22264-50-2 -
![1841081-72-8 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride 98+](https://img.chemicalbook.in//Content/image/CP5.jpg) 1841081-72-8 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride 98+View Details
1841081-72-8 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride 98+View Details
1841081-72-8