1,1,2,2-Tetrabromoethane
Synonym(s):1,1,2,2-Tetrabromoethane;Acetylene tetrabromide;Muthmanns liquid;TBE;TBE, Muthmanns liquid, Acetylene tetrabromide
- CAS NO.:79-27-6
- Empirical Formula: C2H2Br4
- Molecular Weight: 345.65
- MDL number: MFCD00000133
- EINECS: 201-191-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
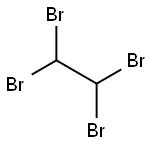
What is 1,1,2,2-Tetrabromoethane?
Chemical properties
yellowish heavy liquid with odour of camphor and
Chemical properties
Acetylene tetrabromide is a combustible, colorless to yellow liquid. Strong odor.
Physical properties
Colorless to pale yellow liquid with a pungent odor resembling camphor and iodoform
The Uses of 1,1,2,2-Tetrabromoethane
1,1,2,2-Tetrabromoethane was used to prepare tribromoethene by dehydrohalogenation using sodium hydroxide in methanol.
The Uses of 1,1,2,2-Tetrabromoethane
Heavy liquid used for the separation of minerals
The Uses of 1,1,2,2-Tetrabromoethane
In microscopy, as solvent, separating minerals by density.
The Uses of 1,1,2,2-Tetrabromoethane
Gauge fluid; solvent; refractive index liquid in microscopy
General Description
A yellowish liquid with a pungent odor, much like camphor. Irritates skin. Ingestion or inhalation may produce irritation or a narcotic effect. Chronic exposure may damage liver. Noncombustible, but decomposes at 374°F, releasing flammable and toxic fumes. Much denser than water. Used as a solvent for fats, oils and waxes.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,1,2,2-Tetrabromoethane is incompatible with strong bases and chemically active metals. Incompatible with hot iron, aluminum and zinc in the presence of steam. May react with magnesium. Softens or destroys most plastics and rubber .
Health Hazard
Inhalation of vapors or dust is extremely irritating. May cause burning of eyes and flow of tears. May cause coughing, difficult breathing and nausea. Brief exposure effects last only a few minutes. Exposure in an enclosed area may be very harmful. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Some of these materials may burn, but none ignite readily. Containers may explode when heated.
Safety Profile
Poison by inhalation, ingestion, and intraperitoneal routes. An eye and skin irritant and a narcotic. Questionable carcinogen with experimental neoplastigenic data. Mutation data reported. When heated it emits hghly toxic fumes of carbonyl bromide and Br-. See also ACETYLENE COMPOUNDS and BROMIDES
Potential Exposure
Acetylene tetrabromide is used as a solvent; a gauge fluid, and as a refractive index liquid in microscopy.
Shipping
UN2504 Tetrabromoethane, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Wash it successively with conc H2SO4 (three times) and H2O (three times), dry it with K2CO3 and CaSO4 and distil it in a vacuum or at ~760mm. [Beilstein 1 IV 162.]
Incompatibilities
Chemically active metals (sodium, potassium, magnesium, and zinc), strong caustics; hot iron. Contact with strong oxidizers may cause fire and explosions.
Waste Disposal
Incineration in admixture with combustible fuel and with scrubber to remove halo acids produced.
Properties of 1,1,2,2-Tetrabromoethane
| Melting point: | 1 °C |
| Boiling point: | 244 °C |
| Density | 2.967 g/mL at 25 °C(lit.) |
| vapor density | 11.9 (vs air) |
| vapor pressure | 0.1 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 118-120°C/15mm |
| storage temp. | Store at +15°C to +25°C. |
| solubility | alcohol: miscible |
| form | Liquid |
| color | Clear |
| Water Solubility | 0.63 g/L (20 ºC) |
| Merck | 14,9185 |
| BRN | 1098321 |
| Henry's Law Constant | 6.40 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | NIOSH REL: IDLH 8 ppm; OSHA PEL: 1 ppm (14 mg/m3). |
| Dielectric constant | 5.6(Ambient) |
| Stability: | Stable. Incompatible with strong oxidizing agents, aluminium, magnesium, alkali metals. |
| CAS DataBase Reference | 79-27-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,1,2,2-Tetrabromoethane(79-27-6) |
| EPA Substance Registry System | 1,1,2,2-Tetrabromoethane (79-27-6) |
Safety information for 1,1,2,2-Tetrabromoethane
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,1,2,2-Tetrabromoethane
1,1,2,2-Tetrabromoethane manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Acetylene tetrabromide, 99% CAS 79-27-6View Details
Acetylene tetrabromide, 99% CAS 79-27-6View Details
79-27-6 -
 1,1,2,2-Tetrabromoethane CAS 79-27-6View Details
1,1,2,2-Tetrabromoethane CAS 79-27-6View Details
79-27-6 -
 1,1,2,2-Tetrabromoethane CAS 79-27-6View Details
1,1,2,2-Tetrabromoethane CAS 79-27-6View Details
79-27-6 -
 Acetylene tetrabromide 98% ( 1,1,2,2-Tetrabromoethane ) CAS 79-27-6View Details
Acetylene tetrabromide 98% ( 1,1,2,2-Tetrabromoethane ) CAS 79-27-6View Details
79-27-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8