CHLOROETHANE
Synonym(s):Chloroethane;Chloroethane solution;Ethyl chloride
- CAS NO.:75-00-3
- Empirical Formula: C2H5Cl
- Molecular Weight: 64.51
- MDL number: MFCD00000961
- EINECS: 200-830-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
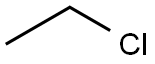
What is CHLOROETHANE?
Description
Ethyl chloride is a colorless gas or liquid(below 12℃) with a pungent, ether-like odor and a burningtaste. Shipped as a liquefied compressed gas. Molecularweight 5 64.52; Specific gravity (H2O:1) = 0.92 (liquid at0℃); Boiling point = 12.2℃; Freezing/Meltingpoint = 2138.9; Vapor pressure = 1000 mmHg at 20℃;Flash point = -50℃ (liquid); Autoignitiontemperature = 519℃. Explosive limits: LEL = 3.8%;UEL = 15.4%. Hazard Identification (based on NFPA-704M Rating System): Health 1, Flammability 4, Reactivity 0.Slightly soluble in water; solubility = 0.6%.
Background
Ethyl chloride, or chloroethane, has a chemical formula C2H5Cl. It was commonly used in the production of tetraethyllead (TEL), which is an additive for gasoline. It was also used in other commerical applications as a chemical reagent. It is still used in the treatment of cellulose to make ethylcellulose for commercial products. Ethyl chloride is used as a diagnostic tool to detect a dead tooth with nonviable pulp.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit
The Uses of CHLOROETHANE
Historically, chloroethane was used to make tetraethyl lead (an anti-knock additive to leaded gasoline). Presently, chloroethane is widely used as a blower in foamed plastics. The use of chloroethane in insecticides and as an ethylating agent in the manufacture of dyes and drugs, refrigerants, local anesthetics, paints, chemicals, and pharmaceuticals has also been approved. In medicine, chloroethane is used to reduce pain caused by burns and insect bites, test pulp freshness in dentistry, and as a supplement in treating alopecia areata and creep eruptions as an anti-stimulant to relieve muscle and visceral pain.
Health effect
Inhalation of high concentrations of chloroethane sensitizes the heart to the effects of catecholamines. This, together with ethyl chloride-induced euphoria, excitement, asphyxia, and hypoxia can result in arrhythmias and death. It has been proposed that chloroethane may depress atrioventricular nodal conduction, causing atrioventricular block.
Storage
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. Priorto working with this chemical you should be trained on itsproper handling and storage. Before entering confined spacewhere this chemical may be present, check to make surethat an explosive concentration does not exist. Ethyl chloride must be stored to avoid contact with oxidizers (such asperoxides, chlorates, perchlorates, nitrates, and permanganates) or chemically active metals (such as sodium, potassium, calcium, powdered aluminum, zinc, and magnesium)because violent reactions occur. Store in tightly closed containers in a cool, well-ventilated area away from heat.Sources of ignition, such as smoking and open flames, areprohibited where ethyl chloride is handled, used, or stored.Metal containers used in the transfer of 5 gallons or more ofethyl chloride should be grounded and bonded. Drums mustbe equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof ethyl chloride. Procedures for the handling, use, and storage of cylinders should be in compliance with OSHA1910.101 and 1910.169, as with the recommendations ofthe Compressed Gas Association.
Synthesis
Chloroethane is synthesized using ethylene hydrochloride and as a by-product of polyvinyl chloride production.
Properties of CHLOROETHANE
| Melting point: | −139 °C(lit.) |
| Boiling point: | 12.3 °C(lit.) |
| Density | 0.89 g/mL at 25 °C(lit.) |
| vapor density | 2.22 (vs air) |
| vapor pressure | 32.29 psi ( 55 °C) |
| refractive index | 1.3676 |
| Flash point: | <−30 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol, ether (U.S. EPA, 1985); miscible with chlorinated hydrocarbons such as
chloroform, carbon tetrachloride, and tetrachloroethane. |
| form | Colorless gas |
| color | Colorless to Almost colorless |
| Odor | Ethereal; pungent, ethereal; ether-like. |
| Water Solubility | 5.074g/L(20 ºC) |
| Merck | 14,3782 |
| Henry's Law Constant | 7.59, 9.58, 11.0, 12.1(x 10-3 atm?m3/mol), and 14.3 at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth et al.,
1988) |
| Exposure limits | TLV-TWA 1000 ppm (~2600 mg/m3)
(ACGIH, MSHA, NIOSH, and OSHA); IDLH
20,000 ppm (NIOSH). |
| Stability: | Stable. Highly flammable - may form explosive mixtures with air. Incompatible with strong oxidizing agents, alkali metals and their alloys. |
| CAS DataBase Reference | 75-00-3(CAS DataBase Reference) |
| IARC | 3 (Vol. 52, 71) 1999 |
| EPA Substance Registry System | Chloroethane (75-00-3) |
Safety information for CHLOROETHANE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Gas Cylinder Compressed Gases GHS04  Health Hazard GHS08 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure H351:Carcinogenicity H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for CHLOROETHANE
CHLOROETHANE manufacturer
Sainor Laboratories Pvt Ltd Unit III
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Ethyl Chloride 75-00-3 98%View Details
Ethyl Chloride 75-00-3 98%View Details
75-00-3 -
 75-00-3 Chloroethane 98%View Details
75-00-3 Chloroethane 98%View Details
75-00-3 -
 Chloroethane (ca. 17% in Ethyl Ether, ca. 2.0mol/L) CAS 75-00-3View Details
Chloroethane (ca. 17% in Ethyl Ether, ca. 2.0mol/L) CAS 75-00-3View Details
75-00-3 -
 Ethyl Chloride 25% 1,4 Dioxane CAS 75-00-3View Details
Ethyl Chloride 25% 1,4 Dioxane CAS 75-00-3View Details
75-00-3 -
 Ethyl Chloride 25% Toluene CAS 75-00-3View Details
Ethyl Chloride 25% Toluene CAS 75-00-3View Details
75-00-3 -
 Chloroethane solution CAS 75-00-3View Details
Chloroethane solution CAS 75-00-3View Details
75-00-3 -
 Chloroethane solution CAS 75-00-3View Details
Chloroethane solution CAS 75-00-3View Details
75-00-3 -
 75-00-3 Chloroethane 5.0M / 25% Toluene /25% 1,4 Dioxane 25% & 5.0MView Details
75-00-3 Chloroethane 5.0M / 25% Toluene /25% 1,4 Dioxane 25% & 5.0MView Details
75-00-3
