79-27-6
Product Name:
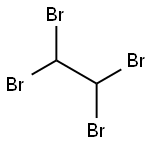
1,1,2,2-Tetrabromoethane
Formula:
C2H2Br4
Synonyms:
1,1,2,2-Tetrabromoethane;Acetylene tetrabromide;Muthmanns liquid;TBE;TBE, Muthmanns liquid, Acetylene tetrabromide
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Acetylene tetrabromide appears as a yellowish liquid with a pungent odor, much like camphor. Irritates skin. Ingestion or inhalation may produce irritation or a narcotic effect. Chronic exposure may damage liver. Noncombustible, but decomposes at 374 °F, releasing flammable and toxic fumes. Much denser than water. Used as a solvent for fats, oils and waxes. |
|---|---|
| Color/Form | Yellowish, heavy liquid /SRP: technical grade/ |
| Odor | Odor of camphor and iodoform |
| Boiling Point | 471 °F at 760 mmHg (NTP, 1992) |
| Melting Point | 30 °F (NTP, 1992) |
| Flash Point | 635 °F (NFPA, 2010) |
| Solubility | less than 1 mg/mL at 68 °F (NTP, 1992) |
| Density | 2.9656 at 68 °F (NTP, 1992) - Denser than water; will sink |
| Vapor Density | 11.9 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.1 mmHg at 68 °F ; 1 mmHg at 149 °F (NTP, 1992) |
| LogP | 2.8 |
| Stability/Shelf Life | Heat /contributes to instability/. |
| Autoignition Temperature | 635 °F (NTP, 1992) |
| Decomposition | NONCOMBUSTIBLE BUT DECOMPOSES @ 374 °F (190 °C) TO LIBERATE FLAMMABLE & HIGHLY TOXIC VAPORS. /SRP: EG, HYDROGEN BROMIDE AND CARBON MONOXIDE/ |
| Corrosivity | ... Will attack some forms of plastics, rubber, and coatings. |
| Heat of Vaporization | 48.7 kJ/mol at boiling point |
| Surface Tension | 49.67 dynes/cm at 20 °C (in contact with air) |
| Refractive Index | Index of refraction: 1.6353 @ 20 °C/D |
| Kovats Retention Index | 1206 1226 1245 1265 1268.5 1269 |
| Other Experimental Properties | 1 mg/l = 70.4 ppm and 1 ppm = 14 mg/cu m @ 25 °C |
| Chemical Classes | Solvents -> Brominated Solvents |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 345.65 g/mol |
|---|---|
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 1 |
| Exact Mass | 345.68490 g/mol |
| Monoisotopic Mass | 341.68900 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 26.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Acetylene tetrabromide appears as a yellowish liquid with a pungent odor, much like camphor. Irritates skin. Ingestion or inhalation may produce irritation or a narcotic effect. Chronic exposure may damage liver. Noncombustible, but decomposes at 374 °F, releasing flammable and toxic fumes. Much denser than water. Used as a solvent for fats, oils and waxes.