1,1'-Azobis(cyanocyclohexane)
Synonym(s):1,1′-Azobis(cyanocyclohexane);ACHN
- CAS NO.:2094-98-6
- Empirical Formula: C14H20N4
- Molecular Weight: 244.34
- MDL number: MFCD00046334
- EINECS: 218-254-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
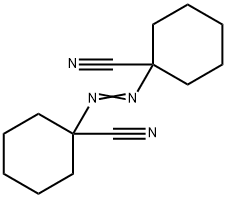
What is 1,1'-Azobis(cyanocyclohexane)?
Chemical properties
faintly beige crystalline powder
The Uses of 1,1'-Azobis(cyanocyclohexane)
A more efficient radical initiator than AIBN has been employed to initiate primary radical reactions.
What are the applications of Application
1,1′-Azobis(cyclohexanecarbonitrile) is a radical initiator
Synthesis Reference(s)
Journal of the American Chemical Society, 71, p. 2661, 1949 DOI: 10.1021/ja01176a018
Organic Syntheses, Coll. Vol. 4, p. 66, 1963
General Description
A white to light colored solid substance. Insoluble in water and more dense that water. May cause irritation to skin, eyes, and mucous membranes. May be toxic by ingestion. If exposed to high temperatures or flames 1,1'-Azobis(cyanocyclohexane) may ignite and burn with an intense flame. Dust may form an explosive mixture in air.
Air & Water Reactions
Dust may form an explosive mixture in air. Insoluble in water.
Reactivity Profile
Self-decomposition or self-ignition may be triggered by heat, chemical reaction, friction or impact. May be ignited by heat, sparks or flames. 1,1'-Azobis(cyanocyclohexane) is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides.
Health Hazard
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.
Purification Methods
Purify the nitrile by dissolving it in boiling 95%EOH as rapidly as possible, cool overnight at 0o, filter, wash with a little EtOH and dry it in a vacuum desiccator over CaCl2. Note that prolonged heating >80o causes decomposition. Recrystallise it from EtOH. It should be regarded as potentially explosive. It is a radical initiator. [Overberger et al. Org Synth Coll Vol IV 66 1963, Beilstein 16 II 97.]
Properties of 1,1'-Azobis(cyanocyclohexane)
| Melting point: | 114-118 °C(lit.) |
| Boiling point: | 423.9±38.0 °C(Predicted) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| vapor pressure | 0-0.001Pa at 20-25℃ |
| storage temp. | 2-8°C |
| form | Solid:crystalline |
| BRN | 960744 |
| Stability: | Stable, but decomposes exothermically above about 114 C |
| CAS DataBase Reference | 2094-98-6(CAS DataBase Reference) |
| EPA Substance Registry System | 1,1'-Azobiscyclohexanecarbonitrile (2094-98-6) |
Safety information for 1,1'-Azobis(cyanocyclohexane)
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H242:Self-reactive substances and mixtures; and Organic peroxides H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P235:Keep cool. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P403:Store in a well-ventilated place. |
Computed Descriptors for 1,1'-Azobis(cyanocyclohexane)
1,1'-Azobis(cyanocyclohexane) manufacturer
Syglak Laboratories Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2094-98-6 1,1'-Azodicyclohexanecarbonitrile 98%View Details
2094-98-6 1,1'-Azodicyclohexanecarbonitrile 98%View Details
2094-98-6 -
 1,1′-Azobis(cyclohexanecarbonitrile) CAS 2094-98-6View Details
1,1′-Azobis(cyclohexanecarbonitrile) CAS 2094-98-6View Details
2094-98-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8