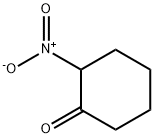
NITROCYCLOHEXANE
- CAS NO.:1122-60-7
- Empirical Formula: C6H11NO2
- Molecular Weight: 129.16
- MDL number: MFCD00003843
- EINECS: 214-354-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 16:37:02
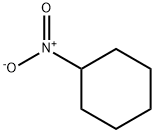
What is NITROCYCLOHEXANE?
Chemical properties
Colorless to dark yellow liquid
Chemical properties
Nitrocyclohexane is a highly flammable, colorless liquid.
Definition
ChEBI: A C-nitro compound that is cyclohexane in which a single hydrogen is replaced by a nitro group.
Synthesis Reference(s)
Journal of the American Chemical Society, 75, p. 4047, 1953 DOI: 10.1021/ja01112a049
Tetrahedron, 46, p. 7443, 1990 DOI: 10.1016/S0040-4020(01)89059-1
Tetrahedron Letters, 21, p. 1117, 1980 DOI: 10.1016/S0040-4039(01)83928-9
General Description
Colorless liquid.
Air & Water Reactions
Highly Flammable
Reactivity Profile
Nitroalkanes, such as NITROCYCLOHEXANE, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive. Nitroalkanes are insoluble in water. Flammable/combustible material. May be ignited by heat, sparks or flames.
Potential Exposure
Used in organic synthesis.
Shipping
UN3382 Toxic by inhalation liquid, n.o.s. with an LC50 lower than or equal to 1000 ml/m3 and saturated vapor concentration ≥10 LC50, Hazard Class: 6.1; Labels: 6.1 Technical Name Required, Inhalation Hazard Zone B.
Incompatibilities
A nitro compound; a fire and explosive hazard. May form explosive mixture with air. Nitrocyclohexane, a nitroalkane, is a strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive. Incompatible with alkalis, and metal oxides. This chemical is highly reactive and may be heat-and shock-sensitive.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of NITROCYCLOHEXANE
| Melting point: | −34 °C(lit.) |
| Boiling point: | 205.5-206 °C768 mm Hg(lit.) |
| Density | 1.061 g/mL at 25 °C(lit.) |
| vapor density | 4.46 (vs air) |
| vapor pressure | 768 mm Hg ( 205 °C) |
| refractive index | n |
| Flash point: | 166 °F |
| solubility | soluble in Alcohol |
| pka | 8.44±0.20(Predicted) |
| form | clear liquid |
| color | Light orange to Yellow to Green |
| EPA Substance Registry System | Nitrocyclohexane (1122-60-7) |
Safety information for NITROCYCLOHEXANE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for NITROCYCLOHEXANE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 Nitrocyclohexane CAS 1122-60-7View Details
Nitrocyclohexane CAS 1122-60-7View Details
1122-60-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
