1-Naphthyl isothiocyanate
- CAS NO.:551-06-4
- Empirical Formula: C11H7NS
- Molecular Weight: 185.24
- MDL number: MFCD00003882
- EINECS: 208-990-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
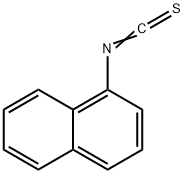
What is 1-Naphthyl isothiocyanate?
Description
1-Napthylisothiocyanate is a toxic chemical that has been used to create experimental cholestasis in rodents. Laboratory studies have also shown that 1-napthylisothiocyanate can act as a chemoprotectant in rodents challenged with a tumorinducing agent such as phorbol-12 myristate 13-acetate.
Chemical properties
white crystalline powder
The Uses of 1-Naphthyl isothiocyanate
1-Naphthyl isothiocyanate (NITC) can be used as a source of thiourea moiety to synthesize derivatives of calix[4]arenes to be used as anion acceptors. It can also be used for the synthesis of naphthyl substituted 1,2,4-triazoles and 1,3,4-thiadiazoles.
The Uses of 1-Naphthyl isothiocyanate
1-Naphthylisothiocyanate is used as an ingredient in insecticides. It is also found in cyanamide, which is used in many industrial applications. It is commonly used in medical and drug studies in animals as a model hepatotoxicant producing experimental cholestasis in rats and mice.
The Uses of 1-Naphthyl isothiocyanate
1-Naphthyl isothiocyanate is a tool for the study of liver damage which causes bile stasis and hyperbilirubinemia acutely and bile duct hyperplasia and biliary cirrhosis chronically, with changes in hepatocyte function.
What are the applications of Application
1-Naphthyl isothiocyanate is a tool for the study of liver damage
Definition
ChEBI: 1-naphthyl isothiocyanate is an isothiocyanate. It has a role as an insecticide. It derives from a hydride of a naphthalene.
General Description
Odorless white needles or powder. Tasteless.
Air & Water Reactions
1-Naphthyl isothiocyanate is moisture sensitive. . Undergoes slow hydrolysis. Insoluble in water.
Reactivity Profile
Isocyanates and thioisocyanates, such as 1-Naphthyl isothiocyanate, are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence, [Wischmeyer(1969)].
Fire Hazard
Flash point data for 1-Naphthyl isothiocyanate is not available, but 1-Naphthyl isothiocyanate is probably combustible.
Environmental Fate
Naphthylisothiocyanate is moisture sensitive and insoluble in water. The hydrolysis rate is slow.
Purification Methods
Crystallise the isothiocyanate from hexane (1g in 9 mL). It forms white needles and is soluble in most organic solvents but is insoluble in H2O. It is absorbed through the skin and may cause dermatitis. [Cymmerman-Craig et al. Org Synth Coll Vol IV 700 1963, Beilstein 12 H 1244, 12 III 2948.]
Toxicity evaluation
1-Naphthylisothiocyanate causes separation of extracellular tight junctions that seal bile canaliculi, impairing bile formation. 1-Naphthylisothiocyanate inhibits microsomal drug-metabolizing activity. In rats, 1-naphthylisothiocyanteglutathione complex is formed in hepatocytes, then transported to the bile ducts, where it is released from the glutathione, and then selectively injures biliary epithelial cells, resulting in reduced bile flow.
Properties of 1-Naphthyl isothiocyanate
| Melting point: | 55.5-57 °C(lit.) |
| Boiling point: | 190 °C(Press: 20 Torr) |
| Density | 1.1142 (rough estimate) |
| refractive index | 1.5500 (estimate) |
| storage temp. | 2-8°C |
| form | Fine Crystalline Powder |
| color | Yellow |
| Water Solubility | insoluble |
| Sensitive | Moisture Sensitive |
| Merck | 14,6411 |
| BRN | 637868 |
| Stability: | Stable, but moisture sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 551-06-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Naphthalene, 1-isothiocyanato-(551-06-4) |
| EPA Substance Registry System | 1-Naphthylisothiocyanate (551-06-4) |
Safety information for 1-Naphthyl isothiocyanate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-Naphthyl isothiocyanate
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran

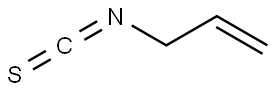
You may like
-
 1-Naphthyl isothiocyanate CAS 551-06-4View Details
1-Naphthyl isothiocyanate CAS 551-06-4View Details
551-06-4 -
 1-Naphthyl Isothiocyanate CAS 551-06-4View Details
1-Naphthyl Isothiocyanate CAS 551-06-4View Details
551-06-4 -
 1-Naphthyl isothiocyanate CAS 551-06-4View Details
1-Naphthyl isothiocyanate CAS 551-06-4View Details
551-06-4 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5