1-(1-NAPHTHYL)-2-THIOUREA
Synonym(s):N-(1-Naphthyl)thiourea
- CAS NO.:86-88-4
- Empirical Formula: C11H10N2S
- Molecular Weight: 202.28
- MDL number: MFCD00041824
- EINECS: 201-706-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
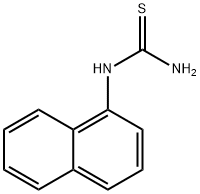
What is 1-(1-NAPHTHYL)-2-THIOUREA?
Description
a-Naphthylthiourea (ANTU; also called DIRAX) is toxic by inhalation, ingestion, or skin contact. Due to its very narrow spectrum of activity, production was discontinued a long time ago. Exposure to ANTU causes pulmonary edema; therefore, it is often used as an experimental pneumotoxin. ANTU is a graycolored, prism-shaped, odorless powder with a bitter taste. It has a molecular weight of 202.28 and melting point of 200 ℃ and does not ignite readily. However, on interaction with potential oxidizing agents, it may cause fire and explosions, which can lead to hazardous decomposition products such as sulfur dioxide, nitrogen dioxide, and carbon monoxide. The structure of ANTU contains the naphthalene chromophore, which absorbs UV light at ~311 nm. This suggests that ANTU can undergo photolysis.
Chemical properties
greyish to beige-brown crystals or cryst. powder
Chemical properties
Alpha-naphthylthiourea (α-naphthalene thiourea) is a pure white or beige-brown solid/ blue-gray powder. It is hard to dissolve in water, acid, and general organic solvents, but dissolves in boiling ethanol and alkaline solution. On decomposition, ANTU releases carbon monoxide, toxic and irritating fumes and gases, and carbon dioxide. It is a rodenticide and a poison bait to lure rodents.
Chemical properties
ANTU is a noncombustible, white crystalline solid or gray powder. Odorless.
Physical properties
Colorless crystals when pure. Technical product is grayish-blue. Odorless solid. Bitter taste.
The Uses of 1-(1-NAPHTHYL)-2-THIOUREA
Rodenticide. Specific control for the adult Norway rat; less toxic to other rat species.
The Uses of 1-(1-NAPHTHYL)-2-THIOUREA
ANTU an organosulfur is a derivative of thiourea. It is a singledose rodenticide that is specifically used against Norway rats as a bait. However, it is futile against all other species of rodents. Because of its tendency to cause resistance and specificity only toward Norway rodents, this poison rapidly lost popularity and is no longer manufactured in the United States.
Definition
ChEBI: ANTU is a member of naphthalenes.
General Description
White crystal or powder; technical product is gray powder. Has no odor but a bitter taste. Used primarily as a rodenticide for control of adult Norway rats. Not produced commercially in the U.S.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
1-(1-NAPHTHYL)-2-THIOUREA is incompatible with the following: Strong oxidizers, silver nitrate .
Hazard
Toxic by ingestion
Health Hazard
Moderately toxic: probable oral lethal dose (human) 0.5-5 gm/kg, or between 1 ounce and 1 pint (or l lb.) for 150 lb. person. Chronic sublethal exposure may cause antithyroid activity. Can produce hyperglycemia of three times normal in three hours. People with chronic respiratory disease or liver disease may be especially at risk.
Health Hazard
α-Naphthalene thiourea, a rodenticide, is very toxic and is fatal if swallowed. Exposures to ANTU cause poisoning with symptoms that include, but are not limited to, headache, weakness, dizziness, shortness of breath, cyanosis, blood abnormalities, methemoglobinemia, irritation of the digestive tract, liver and kidney damage, cardiac and CNS disturbances, convulsions, tachycardia, dyspnea, vertigo, tinnitus, weakness, disorientation, lethargy, drowsiness, and fi nally coma and death. The target organs include the blood, kidneys, CNS, liver, lungs, cardiovascular system, and blood-forming organs.
Fire Hazard
Emits sulfur dioxide, oxides of nitrogen, and carbon monoxide fumes upon decomposition. 1-(1-NAPHTHYL)-2-THIOUREA reacts with silver nitrate and strong oxidizers. Avoid decomposing heat.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic to humans by an unspecified route. Questionable carcinogen with experimental tumorigenic data. Mutagenic data. A rodenticide used extensively. Death is caused by pulmonary edema. Chronic toxicity has been known to cause dermatitis and a decrease in the white blood cells. When heated to decomposition it emits toxic fumes of NOx and SOx.
Potential Exposure
ANTU or its formulations are used as a rodenticide.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24°48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
ANTU was not carcinogenic in rodent feeding studies.4 Cases of bladder tumors among rat catchers exposed to ANTU have been attributed to b-naphthylamine, a manufacturing impurity of ANTU. In bacterial assays ANTU induced mutations.
Environmental Fate
Chemical/Physical. The hydrolysis rate constant for ANTU at pH 7 and 25°C was
determined to be 8 × 10–5/hour, resulting in a half-life of 361 days (Ellington et al., 1988)
Emits very toxic fumes of nitrogen and sulfur oxides when heated to decomposition
(Lewis, 1990)
Storage
α-Naphthalene thiourea should be kept stored in a tightly closed container in a locked poison room, in a cool, dry, well-ventilated area away from incompatible substances.
Shipping
UN1651 Naphthylthiourea, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise ANTU from EtOH. [Beilstein 12 III 2941, 12 IV 3086.]
Toxicity evaluation
ANTU toxicity in the rat is thought to depend on metabolic
activation via the hepatic and lung microsomal enzymes. Two
important metabolites are formed: ANU and atomic sulfur.
ANTU pulmonary toxicity may result, in part, from covalent
binding of sulfur or a metabolite containing carbonyl carbon of
ANTU to macromolecules of liver and lung microsomes. The
covalent binding of atomic sulfur released in the cytochrome
P450 monooxygenase-catalyzed metabolism of thiono-sulfur
compounds is responsible for monooxygenase activity being
inhibited. Damage to liver and possibly lung edema and
neoplasia result from the covalent binding of the electrophilic
S-oxides, S-dioxides or carbene derivatives of these S-oxides
and S-dioxides to tissue macromolecules.
However, it is not known if these metabolites are seen in
humans. ANTU is believed to act on certain enzyme systems
involving the sulfhydryl group similar to other sulfhydryl
inhibitors, such as alloxan, iodoacetamide, and oxophenarsine,
which cause pulmonary edema. Hence the mechanism
of action of causing pulmonary edema from the toxic
effects of these sulfhydryl inhibitors and ANTU is assumed to
be similar. Additionally, ANTU-induced lung damage has
been linked to the formation of oxygen free radicals produced
via the cyclooxygenase pathway. Following exposure to
ANTU, there are a number of biochemical events, such as
alteration in carbohydrate metabolism, adrenal stimulation,
and interaction of the chemical with sulfhydryl groups, but
none of these appear to bear any relationship to the observed
signs of toxicity.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, silver nitrate.
Waste Disposal
Incinerate in a furnace equipped with an alkaline scrubber. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Precautions
Workers should use/handle α-naphthalene thiourea with adequate ventilation. During use, dust generation and accumulation should be minimum, and avoid contact with the eyes, skin, and clothing.
Properties of 1-(1-NAPHTHYL)-2-THIOUREA
| Melting point: | 193 °C |
| Boiling point: | 377.6±25.0 °C(Predicted) |
| Density | 1.333 |
| refractive index | 1.5500 (estimate) |
| storage temp. | Poison room |
| solubility | 4.3 and 86 g/L in acetone and triethylene glycol, respectively (Windholz et al., 1983) |
| form | Crystals or Crystalline Powder |
| pka | 13.12±0.70(Predicted) |
| color | Grayish to beige-brown |
| Odor | bitter taste |
| Water Solubility | 600 mg/L at 20 °C (quoted, Windholz et al., 1983) |
| Merck | 14,722 |
| BRN | 778118 |
| Exposure limits | NIOSH REL: TWA 0.3 mg/m3, IDLH 100 mg/m3; OSHA PEL: TWA
0.3 mg/m3 |
| CAS DataBase Reference | 86-88-4(CAS DataBase Reference) |
| IARC | 3 (Vol. 30, Sup 7) 1987 |
| EPA Substance Registry System | .alpha.-Naphthylthiourea (86-88-4) |
Safety information for 1-(1-NAPHTHYL)-2-THIOUREA
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 1-(1-NAPHTHYL)-2-THIOUREA
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 1-(1-Naphthyl)-2-thiourea CAS 86-88-4View Details
1-(1-Naphthyl)-2-thiourea CAS 86-88-4View Details
86-88-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
