Phenyl isocyanate
Synonym(s):Isocyanatobenzene;PhNCO
- CAS NO.:103-71-9
- Empirical Formula: C7H5NO
- Molecular Weight: 119.12
- MDL number: MFCD00001994
- EINECS: 203-137-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
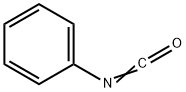
What is Phenyl isocyanate?
Chemical properties
colourless to light yellow liquid with an acrid odour
Chemical properties
Phenyl isocyanate is a colorless liquid with an irritating odor.
The Uses of Phenyl isocyanate
Phenyl Isocyanate is also used in the synthesis of cyanopyridine derivatives in the search for anticancer compounds. It is also used in the synthesis of aminobenzimidazole derivatives with phenylcyclohexyl acetic groups showing antiobesity-antidiabetic activity.
The Uses of Phenyl isocyanate
Phenyl isocyanate was used to prepare chemically modified cellulose paper. It was used in the synthesis of functionalized graphene oxide nanoplatelets.
Definition
ChEBI: An isocyanate composed of a benzene ring bearing a single isocyanato substituent.
Preparation
To a mixture of aniline hydrochloride (12.95 g, 0.1 mol) and dry dioxane (100 mL) was added diphosgene (6.3 mL, 10.4 g, 0.05 mol). The mixture was heated to 60 °C; after stirring for 1.5 h, it became a clear solution. Heating was stopped after 3.5 h and the solvent was removed under reduced pressure. The residue was distilled at 70–73.5 °C (36 mmHg) to give 10.6 g (89%) of phenyl isocyanate. It could be redistilled almost quantitatively, bp 75–77 °C(39 mmHg) or 55–57 °C(16 mmHg).
Synthesis Reference(s)
Journal of the American Chemical Society, 90, p. 3295, 1968 DOI: 10.1021/ja01014a089
General Description
A colorless liquid. About the same density as water. Very toxic by ingestion, inhalation or skin absorption. Very irritating to skin, eyes and mucous membranes. Flash point 132°F.
Air & Water Reactions
Flammable. Decomposes in water.
Reactivity Profile
Isocyanates and thioisocyanates, such as Phenyl isocyanate, are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Polyurethanes are formed by the condensation reaction of diisocyanates with, for example, ethyl glycol. Phenyl isocyanate, cobalt pentamine triazo perchlorate, and nitrosyl perchlorate mixture was stirred for 2-3 minutes. When the stirring was interrupted, the mixture exploded [Chem. Eng. News 46(8):39. 1968].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
A poison. An irritant. Mutation data reported. Flammable liquid when exposed to heat or flame; can react vigorously with oxidizing materials. Has exploded when stirred with (cobalt pentammine triazoperchlorate + nitrosyl perchlorate). When heated to decomposition it emits toxic fumes of CNand NOx. See also CYANATES.
Potential Exposure
Phenyl isocyanate is used as a laboratory reagent and in organic synthesis
Shipping
UN2487 Phenyl isocyanate, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid
Purification Methods
Distil phenylisocyanate under reduced pressure from P2O5. [Beilstein 12 IV 864.]
Incompatibilities
May form explosive mixture with air. Isocyanates are highly flammable and reactive with many compounds, even with themselves. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Reaction with moist air, water or alcohols may form amines and insoluble polyureas and react exothermically, releasing toxic, corrosive or flammable gases, including carbon dioxide; and, at the same time, may generate a violent release of heat increasing the concentration of fumes in the air. Incompatible with amines, aldehydes, alkali metals, ammonia, carboxylic acids, caprolactum, alkaline materials, glycols, ketones, mercaptans, hydrides, organotin catalysts, phenols, strong acids, strong bases, strong reducing agents, urethanes, ureas. Elevated temperatures or contact with acids, bases, tertiary amines, and acyl-chlorides may cause explosive polymerization. Contact with metals may evolve flammable hydrogen gas. Attacks some plastics, rubber and coatings. Contact with metals may evolve flammable hydrogen gas. May accumulate static electrical charges, and may cause ignition of its vapors.
Properties of Phenyl isocyanate
| Melting point: | -30 °C (lit.) |
| Boiling point: | 162-163 °C (lit.) |
| Density | 1.096 g/mL at 25 °C (lit.) |
| vapor pressure | 1.4 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 132 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with ether. |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,7296 |
| BRN | 471391 |
| Dielectric constant | 8.8(20℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, water, moisture, alcohols, amines, strong bases, strong acids. Protect from air. |
| CAS DataBase Reference | 103-71-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, isocyanato-(103-71-9) |
| EPA Substance Registry System | Phenyl isocyanate (103-71-9) |
Safety information for Phenyl isocyanate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Phenyl isocyanate
Phenyl isocyanate manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 Phenyl isocyanate, 99% CAS 103-71-9View Details
Phenyl isocyanate, 99% CAS 103-71-9View Details
103-71-9 -
 Phenyl isocyanate, 99% CAS 103-71-9View Details
Phenyl isocyanate, 99% CAS 103-71-9View Details
103-71-9 -
 Phenyl isocyanate 98 % CAS 103-71-9View Details
Phenyl isocyanate 98 % CAS 103-71-9View Details
103-71-9 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
