Isocyanatocyclohexane
- CAS NO.:3173-53-3
- Empirical Formula: C7H11NO
- Molecular Weight: 125.17
- MDL number: MFCD00003840
- EINECS: 221-639-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
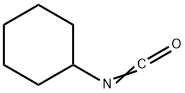
What is Isocyanatocyclohexane?
Description
Cyclohexyl isocyanate is a clear, flammableliquid. It has a sharp, pungent odor. Molecularweight=125.19; Specific gravity (H2O:1): 0.98; Boilingpoint=168℃; Flash point=48℃ (cc). HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 2, Reactivity 0. Soluble in water(reaction).
Chemical properties
colourless or light yellow liquid
Chemical properties
Cyclohexyl isocyanate is a clear, flammable liquid. It has a sharp, pungent odor.
The Uses of Isocyanatocyclohexane
Cyclohexyl Isocyanate reacts with isoquinoline to prepare of Gliquidone through condensation reaction in a solvent under the action of base.
Definition
ChEBI: An isocyanate comprising a cyclohexane core with a single isocyanato substituent.
General Description
A yellowish liquid with an irritating odor. Insoluble in water. Flash point 127°F. Very toxic by inhalation, skin absorption and ingestion. Used to make pharmaceuticals and agricultural chemicals.
Air & Water Reactions
Flammable. Insoluble in water. Reacts slowly with water to form the amine and CO2.
Reactivity Profile
Isocyanates and thioisocyanates are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Poison by intravenous and intraperitoneal routes. Mutation data reported. A flammable liquid when exposed to heat or flame. When heated to decomposition it emits toxic fumes of NOx. See also CYANATES and ESTERS.
Potential Exposure
The material is used in the synthesis of agricultural chemicals.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a corticosteroid spray.
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Color Code—Blue: Health Hazard/Poison: Store in a securepoison location. Prior to working with cyclohexyl isocyanate you should be trained on its proper handling and storage. Cyclohexyl isocyanate must be stored to avoid contactwith moisture and temperatures above 93℃, since violentreactions occur. Store in tightly closed containers in a cool,well-ventilated area away from water, strong bases, alcohol,metal compounds, or surface-active materials. Use onlynonsparking tools and equipment, especially when openingand closing containers of this chemical. Sources of ignition,such as smoking and open flames, are prohibited where thischemical is used, handled, or stored in a manner that couldcreate a potential fire or explosion hazard
Shipping
UN2488 Cyclohexyl isocyanate, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, Inhalation Hazard Zone B.
Incompatibilities
Cyclohexyl isocyanate polymerize due toheating above 93℃/200°F and under the influence of various chemicals including organometallic compounds.Cyclohexyl isocyanate decomposes in fire, forming toxichydrogen cyanide and nitrogen oxides fumes. Reacts withoxidizers, strong bases, water, alcohol, acids, amines, metalcompounds, surface-active materials
Properties of Isocyanatocyclohexane
| Melting point: | -80 °C |
| Boiling point: | 168-170 °C(lit.) |
| Density | 0.98 g/mL at 25 °C(lit.) |
| vapor pressure | 1.83 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 120 °F |
| storage temp. | Flammables area |
| solubility | Chloroform (Sparingly) |
| form | Liquid |
| color | Clear colorless to light amber |
| Odor | sharp pungent odor |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| BRN | 507983 |
| Exposure limits | ACGIH: TWA 25 ppm; STEL 50 ppm OSHA: Ceiling 50 ppm(300 mg/m3) NIOSH: IDLH 200 ppm; Ceiling 50 ppm(300 mg/m3) |
| Stability: | Stability Flammable. Incompatible with water, strong oxidizing agents, alcohols, strong bases, amines, acids, copper alloys, aluminium. May polymerize if exposed to moisture. |
| CAS DataBase Reference | 3173-53-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclohexylisocyanate(3173-53-3) |
| EPA Substance Registry System | Cyclohexyl isocyanate (3173-53-3) |
Safety information for Isocyanatocyclohexane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Isocyanatocyclohexane
Isocyanatocyclohexane manufacturer
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
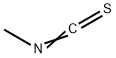
You may like
-
 Cyclohexyl isocyanate 3173-53-3 99%View Details
Cyclohexyl isocyanate 3173-53-3 99%View Details
3173-53-3 -
 Cyclohexyl iso-cyanate, 99% CAS 3173-53-3View Details
Cyclohexyl iso-cyanate, 99% CAS 3173-53-3View Details
3173-53-3 -
 Cyclohexyl isocyanate, 98+% CAS 3173-53-3View Details
Cyclohexyl isocyanate, 98+% CAS 3173-53-3View Details
3173-53-3 -
 Cyclohexyl isocyanate CAS 3173-53-3View Details
Cyclohexyl isocyanate CAS 3173-53-3View Details
3173-53-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
