1-Bromobutane
Synonym(s):1-Bromobutane;Butyl bromide
- CAS NO.:109-65-9
- Empirical Formula: C4H9Br
- Molecular Weight: 137.02
- MDL number: MFCD00000260
- EINECS: 203-691-9
- Update Date: 2025-11-20 17:40:16

What is 1-Bromobutane?
Description
Butyl bromide is a highly flammable, colorlessliquid with a pleasant odor. Molecular weight = 137.04.Boiling point =101.6℃; Specific gravity (H2O:1) = 1.276at 20℃; Freezing/Melting point = 2112.4; Vapor density(air 5 1) = 4.68; Flash point =18℃ (21℃ for sec-isomer).Autoigniton temperature = 265℃. Explosive limits:LEL =2.6% at 100℃; UEL =6.6% at 100℃. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 3, Reactivity 0. Insoluble in water.
Chemical properties
1-bromobutane appears as a clear colorless to slightly yellow liquid with aroma. Flash point 65°F. Denser than water and insoluble in water, soluble in organic solvents such as alcohol, ether and chloroform.Vapors heavier than air.
The Uses of 1-Bromobutane
1-Bromobutane is used as an intermediate in organic synthesis and as a solvent for cleaning and degreasing. It acts as an alkylating agent as well as to prepare organometallic compounds such as n-butyllithium. It is also involved in the synthesis of procaine and tetracaine. It reacts with magnesium metal to prepare the Grignard reagent, which is used to form carbon-carbon bonds.
What are the applications of Application
1-Bromobutane may be used as a derivatization reagent for multiple functional groups (amino, carboxyl, and phenolic hydroxyl groups) of amino acids to improve hydrophobicities and basicities of the amino acids. It may also be used as an internal standard for the quantification of 1- and 2-bromopropane in human urine by headspace gas chromatography.
Synthesis Reference(s)
Journal of the American Chemical Society, 86, p. 964, 1964 DOI: 10.1021/ja01059a073
Organic Syntheses, Coll. Vol. 1, p. 25, 1941
Synthesis, p. 326, 1982
General Description
1-Bromobutane, an alkyl halide, is an alkylating agent. Its rotational constants, nuclear quadrupole constants and centrifugal distortion constants have been stated based on microwave spectral data. The rate coefficient for the reaction of 1-bromobutane with hydrogen atoms has been reported to be 2.4±1.2x1010cm3mol-1s-1.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
1-Bromobutane is incompatible with strong bases and oxidizers [USCG, 1999].
Hazard
Flammable, dangerous fire risk.
Health Hazard
Irritating to the eyes, nose, throat, and upper respiratory tract. Symptoms of exposure include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting. Irritating to the skin.
Safety Profile
Moderately toxic by intraperitoneal route. Mddly toxic by inhalation. Dangerous fire hazard when exposed to heat, flame, or oxidizers. Violent reaction with bromobenzene + sodium above 30℃. Can react with oxidizing materials. To fight fire, use CO2, dry chemical, mist or spray. See also BROMIDES.
Synthesis
1-bromobutane is a primary alkyl halide (primary alkyl) and therefore it is produced from bimolecular nucleophilic substitution reactions (Sn2).
The preparation method of 1-bromobutane is as follows: stirring n-butanol, sodium bromide, water and sulfuric acid evenly, heating under reflux for 3 hours, steaming out the crude bromobutane, washing with water, treating with cold concentrated sulfuric acid, and distilling to obtain the finished product.
Reaction equation: CH3CH2CH2CH2OH+NaBr+H2SO4→CH3CH2CH2CH2Br+Na2SO4+H2O
This halide is easily prepared by reacting butan-1-ol (primary alcohol) with sodium bromide solution and excess of concentrated sulfuric acid. The reaction between sodium bromide and sulphuric acid origins hydrobromic acid.
Potential Exposure
Butyl bromide is used to make otherchemicals and in making pharmaceuticals.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with Butyl bromide you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area. Sources of ignition, such as smoking and open flames, are prohibitedwhere Butyl bromide is handled, used, or stored. Metal containers involving the transfer of 5 gallons or more of Butylbromide should be grounded and bonded. Drums must beequipped with self-closing valves, pressure vacuum bungs,and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers ofButyl bromide.
Shipping
UN1126 & UN23391- & 2-Bromobutane, Hazard Class: 3; Labels: 3—Flammable liquid.
Purification Methods
Wash the bromide with conc H2SO4, water, 10% Na2CO3 and again with H2O. Dry it over CaCl2, CaSO4 or K2CO3, and distil it. Redistil it after drying with P2O5, or pass it through two columns containing 5:1 silica gel/Celite mixture and store it with freshly activated alumina. [Beilstein 1 IV 258.]
Incompatibilities
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. May accumulate static electrical charges and cause ignition of its vapors.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed
Properties of 1-Bromobutane
| Melting point: | -112 °C |
| Boiling point: | 100-104 °C(lit.) |
| Density | 1.276 g/mL at 25 °C(lit.) |
| vapor density | 4.7 (vs air) |
| vapor pressure | 150 mm Hg ( 50 °C) |
| refractive index | n |
| Flash point: | 23 °C |
| storage temp. | Store below +30°C. |
| solubility | 0.6g/l |
| form | Liquid |
| color | Clear colorless to light yellow |
| Odor | characteristic odor |
| explosive limit | 2.8-6.6%, 100°F |
| Water Solubility | 0.608 g/L (30 ºC) |
| Merck | 14,1553 |
| BRN | 1098260 |
| Dielectric constant | 7.1600000000000001 |
| Stability: | Stable. Flammable - note low flash point. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 109-65-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Butane, 1-bromo-(109-65-9) |
| EPA Substance Registry System | 1-Bromobutane (109-65-9) |
Safety information for 1-Bromobutane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-Bromobutane
| InChIKey | MPPPKRYCTPRNTB-UHFFFAOYSA-N |
1-Bromobutane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


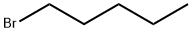
You may like
-
 N-Butyl Bromide 109-65-9 99%View Details
N-Butyl Bromide 109-65-9 99%View Details
109-65-9 -
 1-Bromobutane 99%View Details
1-Bromobutane 99%View Details -
 1-Bromobutane 98%View Details
1-Bromobutane 98%View Details
109-65-9 -
 1-Bromobutane CAS 109-65-9View Details
1-Bromobutane CAS 109-65-9View Details
109-65-9 -
 n-Butyl bromide (Bromobutane) 98% CAS 109-65-9View Details
n-Butyl bromide (Bromobutane) 98% CAS 109-65-9View Details
109-65-9 -
 C4H9Br 1-Bromobutane, Pack Size: 25kg, Packaging Size: 50 kgView Details
C4H9Br 1-Bromobutane, Pack Size: 25kg, Packaging Size: 50 kgView Details
109-65-9 -
 RAW cas 109-65-9 Bromobutane APIView Details
RAW cas 109-65-9 Bromobutane APIView Details
109-65-9 -
 N-Butyl BromideView Details
N-Butyl BromideView Details
109-65-9
