(+)-Usniacin
Synonym(s):(+)-Usnic acid from Usnea dasypoga;2,6-Diacetyl-7,9-dihydroxy-8,9b-dimethyldibenzofuran-1,3(2H,9bH)-dione
- CAS NO.:7562-61-0
- Empirical Formula: C18H16O7
- Molecular Weight: 344.32
- MDL number: MFCD00016878
- EINECS: 231-456-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
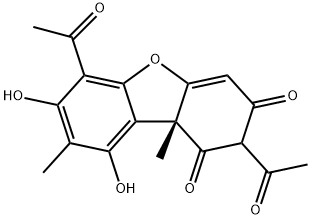
What is (+)-Usniacin?
The Uses of (+)-Usniacin
Usnic acid is used as fragrance; preservative in deodorants; in anti ac ne formulations; antibiotic for topical application.
The Uses of (+)-Usniacin
Usnic Acid acts as an antibacterial and antifungal agent, and are often seen used in cosmetics and facial application chemicals.
The Uses of (+)-Usniacin
(+)-Usnic acid has been used to study the following:
- The mechanism of its antimicrobial activity in bacterial cells.
- Its ability as an antibiofilm agent against Group A Streptococci (GAS).
- Its ability as a potent anti-virulent compound against Candida albicans.
- The mechanism of its toxic effect on hepatocytes.
- Its ability to inhibit the motility of human lung cancer cells.
Definition
ChEBI: (-)-usnic acid is the (-)-enantiomer of usnic acid. It has a role as an EC 1.13.11.27 (4-hydroxyphenylpyruvate dioxygenase) inhibitor. It is a conjugate acid of a (-)-usnic acid(2-). It is an enantiomer of a (+)-usnic acid.
General Description
(+)-Usniacin is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG.
Biological Activity
mic: 0.05 μg/62.5 μl to 3.1 μg/62.5 μlmicroorganisms can colonize a wide variety of medical devices, putting patients at risk for systemic and local infectious complications, including local-site infections, endocarditis, and catheter-related bloodstream infections. (+)-usniacin is a secondary lichen metabolite that possesses antimicrobial activity against various planktonic gram-positive bacteria.
Contact allergens
Usnic acid is a component of lichens, also used as a topical antibiotic. Allergic contact dermatitis from lichens occurs mainly occupationally in forestry and horticultural workers, and in lichen pickers.
in vitro
(+)-usniacin showed antimicrobial activity against the same microorganisms as that of acetone extract. among the three analogues it was the most active one having quite low mic values. furthermore, (+)-usniacin did not show any activity against a. hydrophila and b. cereus whereas (d)-usnic acid did. on the other hand, (+)-usniacin was active against y. enterocolitica whereas (d)-usnic acid was not active [1].
Purification Methods
This very weak acid is the natural form which is recrystallised from Me2CO, MeOH or *C6H6. At 25o it is soluble in H2O (<0.01%), Me2CO (0.77%), EtOAc (0.88%), MeOCH2CH2OH (0.22%) and furfural (7.32%). [Curd & Robertson J Chem Soc 894 1937, Barton & Brunn J Chem Soc 603 1953, resolution: Dean et al. J Chem Soc 1250 1953, synthesis: Barton et al. J Chem Soc 538 1956, Beilstein 18/5 V 586.]
References
[1] tay t, türk ao, yilmaz m, türk h, kivanç m. evaluation of the antimicrobial activity of the acetone extract of the lichen ramalina farinacea and its (+)-usnic acid, norstictic acid, and protocetraric acid constituents. z naturforsch c. 2004 may-jun;59(5-6):384-8.
[2] ghione m, parrello d, grasso l. usnic acid revisited, its activity on oral flora. chemioterapia. 1988 oct;7(5):302-5.
Properties of (+)-Usniacin
| Melting point: | 201-203 °C(lit.) |
| Boiling point: | 399.43°C (rough estimate) |
| alpha | 488 º (c=0.4, CHCl3) |
| Density | 1.2869 (rough estimate) |
| refractive index | 1.4790 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | almost transparency in Chloroform |
| form | powder to crystal |
| pka | 4.00±0.40(Predicted) |
| color | Light orange to Yellow to Green |
| optical activity | [α]25/D +488°, c = 0.7% in chloroform |
| Water Solubility | Partly soluble in water. Soluble in acetone, ethanol, chloroform and furfural. |
| Merck | 14,9893 |
| BRN | 96698 |
| CAS DataBase Reference | 7562-61-0(CAS DataBase Reference) |
Safety information for (+)-Usniacin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for (+)-Usniacin
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 (+)-Usnic Acid CAS 7562-61-0View Details
(+)-Usnic Acid CAS 7562-61-0View Details
7562-61-0 -
 Usnic acid CAS 7562-61-0View Details
Usnic acid CAS 7562-61-0View Details
7562-61-0 -
 (+)-Usnic acid CAS 7562-61-0View Details
(+)-Usnic acid CAS 7562-61-0View Details
7562-61-0 -
 (+)-Usnic acid CAS 7562-61-0View Details
(+)-Usnic acid CAS 7562-61-0View Details
7562-61-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
