Zofenopril
Synonym(s):α-Naphthaleneacetic acid Free acid;1-Naphthaleneacetic acid;1-Naphthaleneacetic acid, Planofix;1-Naphthylacetic acid;NAA
- CAS NO.:81872-10-8
- Empirical Formula: C22H23NO4S2
- Molecular Weight: 429.55
- MDL number: MFCD00870957
- EINECS: 201-705-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-21 22:41:43
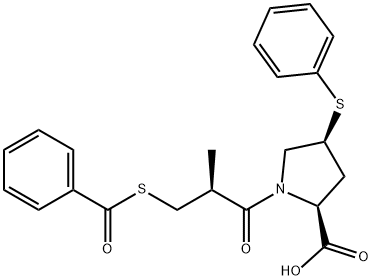
What is Zofenopril?
Originator
Zofenopril Calcium,ZYF Pharm Chemical
The Uses of Zofenopril
Zofenopril-d5 is intended for use as an internal standard for the quantification of Zofenopril by GC- or LC-mass spectrometry.
What are the applications of Application
Zofenopril-d5 Calcium Salt is a compound that is de-esterified into the active inhibitor, Zefenoprilat
Background
Zofenopril is employed as both a cardioprotective and anti-hypertensive agent. It is an angiotensin-converting enzyme (ACE) inhibitor.
Definition
ChEBI: A proline derivative that is 4-(phenylsulfanyl)-L-proline in which the amine proton is replaced by a (2S)-3-(benzoylsulfanyl)-2-methylpropanoyl group. A prodrug for zofenoprilat.
Manufacturing Process
9.9 g (0.031 mole) of cis-4-phenylthio-L-proline is suspended in 100 ml of
water (pH 5.6) and the pH is adjusted to 10.2 by the addition of about 20 ml
of 10% sodium bicarbonate to provide a clear solution. The pH is then
adjusted to 9.5 by the addition of about 4.5 ml of concentrated HCl. The
solution is kept at 30°C while 8.1 g (0.033 mole) of (D)-3-(benzoylthio)-2-
methylpropanoic acid chloride in 30 ml of toluene is added simultaneously
with 100 ml of 10% sodium bicarbonate to keep the pH at 9.3. After about
1/4 of the acid chloride is added, a slimy precipitate begins to form which
persists throughout the reaction. After stirring the reaction mixture at pH 9.3
for 2.5 h, it is made strongly acidic by adding 20% HCl in the presence of
ethyl acetate. The aqueous layer is extracted twice with 350 ml portions of
ethyl acetate and the combined organic layers are washed with 300 ml of
saturated brine and dried (MgSO 4 ). The solvent is removed to yield 11.8 g of
foamy solid cis-1-[D-3-(benzoylthyo)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline hydrochloride.
To a solution of this cis-1-[D-3-(benzoylthyo)-2-methyl-1-oxopropyl]-4-
(phenylthio)-L-proline hydrochloride 11.8 g (0.027 mole) in 70 ml of
acetonitrile there is added about 6.0 g of dicyclohexylamine in 25 ml of ether.
A white crystalline precipitate forms immediately. After standing overnight in
the cold room, the solid is filtered and washed with ether to yield (cis)-1-[D-
3-(benzoylthio)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline,
dicyclohexylamine salt (1:1).
he slightly moist (cis)-1-[D-3-(benzoylthio)-2-methyl-1-oxopropyl]-4-
(phenylthio)-L-proline dicyclohexylamine salt is stirred for 2.5 h in a mixture
of 300 ml of ethyl acetate and 200 ml of 10% potassium bisulfate. Two clear
layers form. The aqueous layer is extracted with two 200 ml portions of ethyl
acetate and the combined organic layers are dried (MgSO 4 ). The solvent is
removed to yield 10.1 g of foamy solid (cis)-1-[D-3-(benzoylthio)-2-methyl-1-
oxopropyl]-4-(phenylthio)-L-proline; melting point 42-44°C.
In practice it is usually used as calcium salt (2:1).
brand name
Zoprace (Bristol-Myers Squibb).
Therapeutic Function
Antihypertensive
Synthesis Reference(s)
The Journal of Organic Chemistry, 33, p. 1675, 1968 DOI: 10.1021/jo01268a091
Metabolism
Not Available
Properties of Zofenopril
| Melting point: | 129-131.5 °C(lit.) |
| Boiling point: | 646.3±55.0 °C(Predicted) |
| alpha | D25 -36.5° (c = 1 in methanol) |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 5 mg/mL (11.64 mM) |
| form | crystalline |
| pka | pK1:4.236 (25°C) |
| color | light yellow |
| CAS DataBase Reference | 81872-10-8(CAS DataBase Reference) |
Safety information for Zofenopril
Computed Descriptors for Zofenopril
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4