ZINC CYANIDE
- CAS NO.:557-21-1
- Empirical Formula: C2N2Zn
- Molecular Weight: 117.43
- MDL number: MFCD00011292
- EINECS: 209-162-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
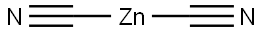
What is ZINC CYANIDE?
Chemical properties
Colorless crystalline solid or white powder. Slight, bitter almond odor. Sinks in water; insoluble.
Physical properties
White powder; orthorhombic crystals; density 1.852 g/cm3; decomposes at 800°C; insoluble in water (about 5mg/L at 20°C); soluble in alkalies, potassium cyanide and ammonia solutions; insoluble in alcohol.
The Uses of ZINC CYANIDE
Zinc cyanide is used in electroplating; as an insecticide; and for separating ammonia from producer gas.
The Uses of ZINC CYANIDE
Zinc cyanide is used in electroplating and as a reagent.
General Description
A white powder. insoluble in water (about 5mg/L at 20°C); soluble in alkalies, potassium cyanide and ammonia solutions; insoluble in alcohol. Toxic by inhalation (dust and the hydrogen cyanide from slight decomposition) and by ingestion. Produces toxic oxides of nitrogen in fires. Used in medicine, in metal plating, and in chemical analysis.
Reactivity Profile
ZINC CYANIDE is decomposed by acids to give off hydrogen cyanide, a flammable poisonous gas. Tends to explosive instability. Capable of violent oxidation under certain condition; fusion with metal chlorates, perchlorates, nitrates or nitrites can cause explosions [Bretherick, 1979 p. 101]. Reacts with incandescence with magnesium [Mellor, 1940, Vol. 4, 271].
Hazard
The compound is toxic by oral and intraperitoneal routes. The intraperitoneal lethal dose in rat is 100 mg/kg.
Health Hazard
EYES: Causes eye burns. SKIN: Irritation. INGESTION OR INHALATION: A bitter, acrid burning taste is sometimes noted followed by a feeling of constriction or numbness in the throat. Salivation and nausea are not unusual, but vomiting is rare. Anxiety, confusion, vertigo, giddiness and often a sensation of stiffness in the lower jaw. Hypernea and dyspnea. Rapid respiration, then slow and irregular. Unconsciousness, convulsions, death from respiratory arrest.
The compound is toxic by oral and intraperitoneal routes. The intraperitoneal lethal dose in rat is 100 mg/kg.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intraperitoneal route. Can react violently with Mg. When heated to decomposition it emits toxic fumes of CN-, ZnO, and NOx. Used in electroplating operations. See also CYANIDE and ZINC COMPOUNDS.
Potential Exposure
Used in pharmaceuticals and medicine. Also used in metal plating, and as a laboratory chemical.
Shipping
UN1713 Zinc cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous material.
Purification Methods
It is a POISONOUS white powder which becomes black on standing if Mg(OH)2 and carbonate are not removed in the preparation. Thus, wash it well with H2O, then well with EtOH, Et2O and dry it in air at 50o. Analyse it by titrating the cyanide with standard AgNO3. Other likely impurities are ZnCl2, MgCl2 and traces of basic zinc cyanide; the first two salts can be washed out. It is soluble in aqueous KCN solutions. However, if purified in this way Zn(CN)2 is not reactive in the Gattermann synthesis. For this, the salt should contain at least 0.33 mols of KCl or NaCl which will allow the reaction to proceed faster. [Adams & Levine J Am Chem Soc 45 2375 1923, Arnold & Sorung J Am Chem Soc 60 1699 1938, Fuson et al. Org Synth Coll Vol III 549 1955.]
Incompatibilities
Releases hydrogen cyanide on contact with moisture including humidity in air. Tends to explosive instability; possible explosion when heated rapidly. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Contact with acids and/or acid salts and alcohols will release highly toxic and flammable hydrogen cyanide gas. Incompatible with reducing agents, alcohols, glycols, combustible materials, ethers, hydrazines, organic substances, metal powders. Capable of violent oxidation under certain condition; fusion with metal chlorates, perchlorates, nitrates or nitrites can cause explosions.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Add strong alkaline hypochlorite and react for 24 hours. Then flush to sewer with large volumes of water.
Properties of ZINC CYANIDE
| Melting point: | 800 °C |
| Density | 1,85 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| solubility | Aqueous Base (Sparingly) |
| form | Powder |
| color | White to off-white |
| Water Solubility | Soluble in alkalies, potassium cyanide and ammonia. Insoluble in water and most solvents. |
| Sensitive | Hygroscopic |
| Merck | 14,10135 |
| BRN | 4124366 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 557-21-1(CAS DataBase Reference) |
| EPA Substance Registry System | Zinc cyanide (557-21-1) |
Safety information for ZINC CYANIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H310:Acute toxicity,dermal H330:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for ZINC CYANIDE
ZINC CYANIDE manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
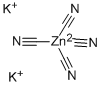
You may like
-
 557-21-1 Zinc Cyanide 99%View Details
557-21-1 Zinc Cyanide 99%View Details
557-21-1 -
 557-21-1 98%View Details
557-21-1 98%View Details
557-21-1 -
 Zinc cyanide 98%View Details
Zinc cyanide 98%View Details
557-21-1 -
 ZINC CYANIDE 99%View Details
ZINC CYANIDE 99%View Details
557-21-1 -
 557-21-1 98%View Details
557-21-1 98%View Details
557-21-1 -
 557-21-1 Zinc cyanide 98%View Details
557-21-1 Zinc cyanide 98%View Details
557-21-1 -
 Zinc cyanide CAS 557-21-1View Details
Zinc cyanide CAS 557-21-1View Details
557-21-1 -
 Zinc Cyanide 99% CAS 557-21-1View Details
Zinc Cyanide 99% CAS 557-21-1View Details
557-21-1
