Zanubrutinib
- CAS NO.:1691249-45-2
- Empirical Formula: C27H29N5O3
- Molecular Weight: 471.55
- MDL number: MFCD31567461
- Update Date: 2024-11-19 20:33:22

What is Zanubrutinib?
Absorption
Following oral administration of zanubrutinib 160 mg twice daily and 320 mg once daily, the mean (%CV) zanubrutinib steady-state concentrations were 2,295 (37%) ng·h/mL and 2,180 (41%) ng·h/mL, respectively. The mean Cmax (%CV) was 314 (46%) ng/mL following 160 mg twice daily and 543 (51%) ng/mL following 320 mg once daily.
The Cmax and AUC of zanubrutinib increase in a dose-proportional manner and there is minimal systemic accumulation after repeated dosing. The median Tmax is 2 hours.
Toxicity
There is limited data on zanubrutinib overdose.
Description
Zanubrutinib, a second-generation BTK inhibitor discovered and developed by BeiGene in China, has been approved by the FDA (in 2019) for treating chronic lymphocytic leukemia (CLL) and certain other indications. Zanubrutinib has lower toxicity and better efficacy than ibrutinib. It is in direct competition with AstraZeneca’s acalabrutinib for the $12 billion blood cancer market currently dominated by the first-in-class BTK inhibitor ibrutinib.
The Uses of Zanubrutinib
Zanubrutinib is classified as a Bruton''s tyrosine kinase inhibitor. Zanubrutinib is a medication for the treatment of adults with mantle cell lymphoma.
Background
Zanubrutinib is a novel Bruton's tyrosine kinase (BTK) inhibitor used for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. Mantle cell lymphoma is an aggressive mature B-cell non-Hodgkin lymphoma associated with early relapse, poor clinical outcomes, and long-term survival. BTK is an enzyme that plays a role in oncogenic signalling pathways, promoting the survival and proliferation of malignant B cells. Compared to the first-generation BTK inhibitor ibrutinib, zanubrutinib displays higher potency and selectivity for BTK with fewer off-target effects. Due to this enhanced selectivity towards BTK, zanubrutinib belongs to the second-generation BTK inhibitor drug group that also includes acalabrutinib, which was approved by the FDA in 2017.
Zanubrutinib was granted accelerated approval by the FDA in November 2019 based on clinical trial results that demonstrated an 84% overall response rate from zanubrutinib therapy in patients with MCL, which measures the proportion of patients in a trial whose tumour is entirely or partially destroyed by a drug. It is currently marketed under the trade name BRUKINSA? and is available as oral capsules. In August 2021, the FDA granted accelerated approval to zanubrutinib for the treatment of adults with Waldenstr?m’s macroglobulinemia. This indication is valid in the US, Europe, and Canada. In September 2021, zanubrutinib was granted another accelerated approval for the treatment of relapsed or refractory marginal zone lymphoma who have received at least one anti-CD20-based regimen. In October 2022, the EMA's Committee for Medicinal Products for Human Use (CHMP) recommended zanubrutinib be granted marketing authorization for the treatment of chronic lymphocytic leukemia.
Indications
Zanubrutinib is indicated for the treatment of:
brand name
BrukinsaTM
General Description
Class: non-receptor tyrosine kinase
Treatment: MCL, MZL, WM
Oral bioavailability = 15%
Elimination half-life = 3.3 h
Protein binding = 94%
Pharmacokinetics
Zanubrutinib is an immunomodulating agent that decreases the survival of malignant B cells. It inhibits BTK by binding to its active site. It works to inhibit the proliferation and survival of malignant B cells to reduce the tumour size in mantle cell lymphoma.
Metabolism
Zanubrutinib is predominantly metabolized by CYP3A4. Its metabolites have not been characterized.
Metabolism
Zanubrutinib showed a mean terminal elimination half-life of approximately 2–4 h (160 or 320 mg, QD) and an estimated oral bioavailability of 15%, relative to 3.9% (fasting state) for ibrutinib. Zanubrutinib is primarily eliminated hepatically via CYP3A4, but its metabolites have not been characterized.
Properties of Zanubrutinib
| Boiling point: | 713.4±60.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:99.67(Max Conc. mg/mL);211.36(Max Conc. mM) DMF:10.0(Max Conc. mg/mL);21.21(Max Conc. mM) DMF:PBS (pH 7.2) (1:5):0.16(Max Conc. mg/mL);0.34(Max Conc. mM) Ethanol:33.33(Max Conc. mg/mL);70.68(Max Conc. mM) |
| form | A solid |
| pka | 15.35±0.40(Predicted) |
| color | White to off-white |
Safety information for Zanubrutinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zanubrutinib
Zanubrutinib manufacturer
Venkatasai Life Sciences
ARRKEM LIFE SCIENCES PRIVATE LIMITED
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
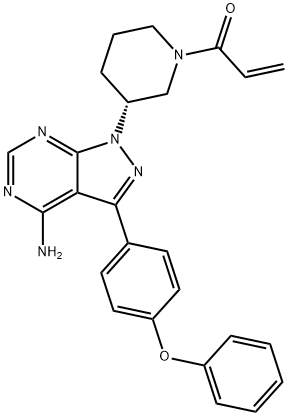
![1-Piperidinecarboxylic acid, 4-[3-(dimethylamino)-1-oxo-2-propen-1-yl]-, 1,1-dim](https://img.chemicalbook.in/CAS/GIF/960201-86-9.gif)
![Propanedinitrile, 2-[hydroxy(4-phenoxyphenyl)Methylene]-](https://img.chemicalbook.in/CAS/20150408/GIF/330792-68-2.gif)
![Propanedinitrile, 2-[Methoxy(4-phenoxyphenyl)Methylene]-](https://img.chemicalbook.in/CAS/20150408/GIF/330792-69-3.gif)
![tert-butyl 4-(3-cyano-2-(4-phenoxyphenyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidin-7-yl)piperidine-1-carboxylate](https://img.chemicalbook.in/CAS/20200331/GIF/2190506-56-8.gif)
You may like
-
 Zanubrutinib 1691249-45-2 99%View Details
Zanubrutinib 1691249-45-2 99%View Details
1691249-45-2 -
![1691249-45-2 (S)-7-(1-acryloylpiperidin-4-yl)-2-(4-phenoxyphenyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide; Zanubrutinib API 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/9a38d8c8-9e26-49d3-ac9a-580a5075ff1c.png) 1691249-45-2 (S)-7-(1-acryloylpiperidin-4-yl)-2-(4-phenoxyphenyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide; Zanubrutinib API 98%View Details
1691249-45-2 (S)-7-(1-acryloylpiperidin-4-yl)-2-(4-phenoxyphenyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide; Zanubrutinib API 98%View Details
1691249-45-2 -
 Zanubritinib 1691249-45-2 98%View Details
Zanubritinib 1691249-45-2 98%View Details
1691249-45-2 -
 Zanubrutinib 1691249-45-2 98%View Details
Zanubrutinib 1691249-45-2 98%View Details
1691249-45-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1