Sunitinib
- CAS NO.:557795-19-4
- Empirical Formula: C22H27FN4O2
- Molecular Weight: 398.47
- MDL number: MFCD09260778
- EINECS: 251-228-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
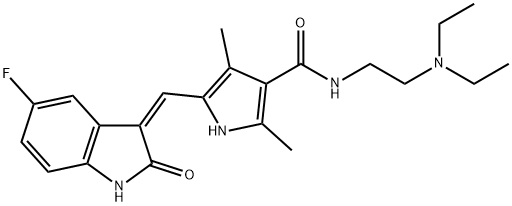
What is Sunitinib?
Absorption
Maximum plasma concentrations (Cmax) of sunitinib are generally observed between 6 and 12 hours (Tmax) following oral administration. Food has no effect on the bioavailability of sunitinib. Sunitinib may be taken with or without food. The pharmacokinetics were similar in healthy volunteers and in the solid tumor patient populations tested, including patients with GIST and RCC.
Toxicity
The maximally tolerated dose for rat, mouse, and dog when given orally is greater than 500 mg/kg. The maximally tolerated dose of a non-human primate is greater 1200 mg/kg.
Description
Sunitinib is an inhibitor of multiple receptor tyrosine kinases (RTKs) involved in tumor proliferation and angiogenesis, including platelet-derived growth factor receptors (PDGFR), vascular endothelial growth factor receptors (VEGFR), and stem cell factor receptor (KIT). It was launched as an oral treatment for gastrointestinal stromal tumors (GIST) and advanced renal-cell carcinoma (RCC). In vitro, sunitib inhibits VEGFR2, PDGFRα, PDGFRβ, KIT, and FLT3 receptors with IC50 values in the 4–14nM range, and the ligand-dependent autophosphorylation of VEGFR2 and PDGFRb with IC50s of approximately 10 nM. In addition, it inhibits the growth of tumor cells expressing dysregulated target RTKs in vitro and inhibits PDGFRb- and VEGFR2-dependent tumor angiogenesis in vivo. Sunitinib exhibits broad and potent antitumor activity, causing regression in murine models of human epidermal (A431), colon (Colo205 and HT-29), lung (NCI-H226 and H460), breast (MDA-MB-435), prostate (PC3-3M-luc), and renal (786-O) cancers, and suppressing or delaying the growth of many others, including the C6 rat and SF763 T human glioma xenografts and B16 melanoma lung cancer.
Chemical properties
Yellow Solid
Originator
Sugen (US)
The Uses of Sunitinib
Sunitinib Malate (Sutent, SU11248) is a multi-targeted RTK inhibitor targeting VEGFR2 (Flk-1) and PDGFRβ with IC50 of 80 nM and 2 nM, and also inhibits c-Kit.
The Uses of Sunitinib
Labelled Sunitinib (S820000), a multi-kinase inhibitor targeting several receptor tyrosine kinases (RTK).
What are the applications of Application
Sunitinib, Free Base is an inhibitor of receptor tyrosine kinases
Indications
Sunitinib is indicated for the following conditions:
Background
Sunitinib is a small-molecule multi-targeted receptor tyrosine kinase (RTK) inhibitor. On January 26, 2006, the agent was formally approved by the US FDA for the indications of treating renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST). For these purposes, sunitinib is generally available as an orally administered formulation. Sunitinib inhibits cellular signaling by targeting multiple RTKs. These include all platelet-derived growth factor receptors (PDGF-R) and vascular endothelial growth factor receptors (VEGF-R). Sunitinib also inhibits KIT (CD117), the RTK that drives the majority of GISTs. In addition, sunitinib inhibits other RTKs including RET, CSF-1R, and flt3.
Definition
ChEBI: Sunitinib is a member of pyrroles and a monocarboxylic acid amide. It has a role as an angiogenesis inhibitor, an antineoplastic agent, an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor, a vascular endothelial growth factor receptor antagonist, an immunomodulator and a neuroprotective agent. It is functionally related to a 3-methyleneoxindole.
brand name
(Pfizer).
Characteristics
Class: receptor tyrosine kinase
Treatment: RCC, GIST
Elimination half-life = 70 h
Protein binding = 95%
Pharmacokinetics
Sunitinib is an oral, small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA on January 26, 2006.
Clinical Use
Tyrosine kinase inhibitor:
Treatment of metastatic renal cell carcinoma
(MRCC), gastrointestinal stromal tumours (GIST)
and pancreatic neuroendocrine tumours (pNET)
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: avoid concomitant use with boceprevir.
Avoid concomitant use with other inhibitors or
inducers of CYP3A4. Dose alterations may be
required.
Metabolism
Sunitinib is metabolized primarily by the cytochrome P450 enzyme, CYP3A4, to produce its primary active metabolite, which is further metabolized by CYP3A4.
Metabolism
Metabolised mainly via the cytochrome P450 isoenzyme
CYP3A4 to its primary active metabolite, which itself is
then further metabolised via CYP3A4.
Elimination is primarily via faeces. In a human mass
balance study of [14C]sunitinib, 61% of the dose was
eliminated in faeces and 16% by the renal route.
References
[1]hui ep1, lui vw, wong cs, ma bb, lau cp, cheung cs, ho k, cheng sh, ng mh, chan at. preclinical evaluation of sunitinib as single agent or in combination with chemotherapy in nasopharyngeal carcinoma. invest new drugs. 2011 dec;29(6):1123-31.
Properties of Sunitinib
| Melting point: | 189-191°C |
| Boiling point: | 572.1±50.0 °C(Predicted) |
| Density | 1.2 |
| Flash point: | 299.8℃ |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 8.5(at 25℃) |
| color | Yellow to Dark Orange |
| CAS DataBase Reference | 557795-19-4(CAS DataBase Reference) |
Safety information for Sunitinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sunitinib
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
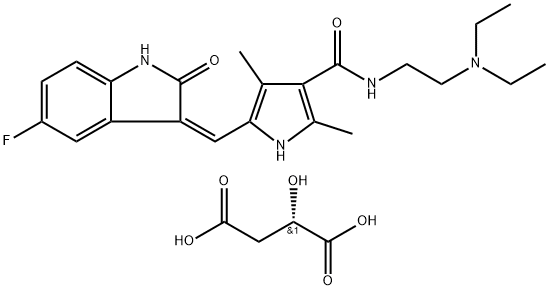
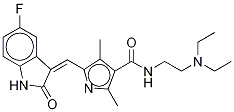
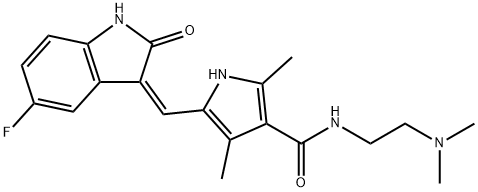


You may like
-
 557795-19-4 Sunitinib 98%View Details
557795-19-4 Sunitinib 98%View Details
557795-19-4 -
 557795-19-4 99%View Details
557795-19-4 99%View Details
557795-19-4 -
 Sunitinib 98%View Details
Sunitinib 98%View Details
557795-19-4 -
 Sunitinib 557795-19-4 98%View Details
Sunitinib 557795-19-4 98%View Details
557795-19-4 -
 557795-19-4 98%View Details
557795-19-4 98%View Details
557795-19-4 -
 Sunitinib Maleate 99%View Details
Sunitinib Maleate 99%View Details -
 Sunitinib CAS 557795-19-4View Details
Sunitinib CAS 557795-19-4View Details
557795-19-4 -
 Sunitinib 95% CAS 557795-19-4View Details
Sunitinib 95% CAS 557795-19-4View Details
557795-19-4
