White phosphorus
- CAS NO.:12185-10-3
- Empirical Formula: P4
- Molecular Weight: 123.896
- MDL number: MFCD03701484
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-22 17:53:22
What is White phosphorus?
Description
White phosphorus,also known as yellow phosphorus, is a nonmetallic element that is found in the form of crystals or a wax-like transparent solid. It ignites spontaneously in air at 86°F, which is also its ignition temperature. White phosphorus should be stored under water and away from heat. It is a dangerous fire risk, with a boiling point of 536°F (280°C) and a melting point of 111°F (43°C). White phosphorus is toxic by inhalation and ingestion, and contact with skin produces burns. The TLV is 0.1 mg/m3 of air, and it is insoluble in water, with a specific gravity of 1.82, which is heavier than air. White phosphorus is shipped and stored under water to keep it from contacting air. The four-digit UN identification number is 2447. The NFPA 704 designation is health 4, flammability 4, and reactivity 2. The primary uses are in rodenticides, smoke screens, and analytical chemistry.
Description
White phosphorus is one of three allotropes of the element phosphorus. The other two are red, an amorphous polymer, and black, a graphitelike polymer. The substance known as yellow phosphorus is actually white phosphorus that contains impurities (e.g., red phosphorus) or that has darkened from exposure to light. Red phosphorus turns violet or purple when it is heated to >550 oC.
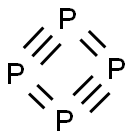
White phosphorus (see images) contains four phosphorus atoms in a tetrahedral arrangement. It has an unpleasant, garliclike odor and is extremely toxic (see hazard information table). It is unstable in air—first forming white fumes before bursting into flames. White phosphorus has been called the “devil’s element” because it glows green in the dark and is pyrophoric.
Because of its instability, white phosphorus is typically stored under water, in which it is barely soluble. The allotrope is soluble in hydrocarbons, carbon disulfide, sulfur chloride (S2Cl2), and other nonpolar solvents.
Molecular phosphorus does not exist in nature; but it is contained in many minerals, primarily hydroxyapatite, fluorapatite, and chorapatite. White phosphorus was discovered in 1669 by Hamburg, Germany, pharmacist/alchemist Hennig Brandt (in some accounts, Brand), who is the subject of the painting The Alchemist Discovering Phosphorus by Joseph Wright. During his search for the mythical philosopher’s stone, Brandt serendipitously produced phosphorus by heating phosphate-containing urine solids with carbonaceous substances. Phosphorus was emitted as a gas (P2), which condensed as a glowing wax.
Brandt did not have an understanding of elements as we know them today. More than 100 years after his discovery, legendary French chemist Antoine Lavoisier recognized phosphorus as an element.
Brandt’s crude process is the basis of modern white phosphorus production. Apatites are mixed with silica (sand) and a carbon source such as coke to produce P2 vapor, which is condensed in water. If fluorapatite is used, the calcium fluoride byproduct can be used to produce fluorine gas.
Worldwide, ≈900,000 t of phosphorus is produced annually. Most of it, ironically, is oxidized back to phosphates for fertilizers (by far the largest use) and the manufacture of pesticides, plasticizers, and animal food additives. The use of white phosphorus itself is limited to ingredients in metallurgy and rodent poisons. It was used as a component of friction matches, until around the turn of the 20th century, when it was replaced by the safer phosphorus sesquisulfide (P4S3).
Chemical properties
Phosphorus is a white to yellow, soft, waxy solid with acrid fumes in air. White/yellow phosphorus is either a yellow or colorless, volatile, crystalline solid which darkens when exposed to light and ignites in air to form white fumes and greenish light. It has a garlic-like odor. Usually shipped or stored in water.
Potential Exposure
White or yellow phosphorus is handled away from air so that exposure is usually limited. Phosphorus was at one time used for the production of matches or “lucifers” but has long since been replaced due to its chronic toxicity. It is used in the manufacture of munitions including tracer bullets, pyrotechnics, explosives, smoke bombs; and other incendiary agents; (because it spontaneously catches fire in air) and as a smoke agent (because it produces clouds of irritating white smoke). Phosphorus is used artificial fertilizers; rodenticides, phosphor bronze alloys; semiconductors, Electro-luminescent coating; and chemicals, such as phosphoric and metallic phosphides. RP is used as a choking/pulmonary agent
Shipping
UN1338 Phosphorus, amorphous, Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN1381 Phosphorus, white, dry; under water, in solution; Phosphorus, yellow, dry; yellow, under water; in solution, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, 6.1Poisonous materials. UN2447 Phosphorus white, molten, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, 6.1-Poisonous materials.
Incompatibilities
Phosphorus, a pyrophoric solid, spontaneously ignite on contact with air, producing toxic phosphorus oxide fumes. Reacts with strong bases, releasing toxic phosphine gas. Phosphorus reacts violently with oxidizers, halogens, some metals, nitrites, sulfur, and many other compounds, causing a fire and explosion hazard. White/yellow reacts with air, halogens, halides, sulfur, oxidizers, alkali hydroxides (forming gas); and metals (forming reactive phosphides). Red is a combustible solid. Friction or contact with oxidizers can cause ignition. Incompatible with many other substances. Forms gas and phosphoric acid on contact with moisture. Opened packages of red phosphorus should be stored under inert gas blanket.
Waste Disposal
Controlled incineration followed by alkaline scrubbing and particulate removal equipment.
Properties of White phosphorus
| Melting point: | 44.1 (0.181mm) [MER06] |
| Boiling point: | 277℃ [CRC10] |
| Density | α: 1.83; β. 1.88 [MER06] |
| solubility | ≈3 mg/l |
| appearance | white, waxy crystalline solid |
| Water Solubility | 1 part/300,000 parts H2O; 1g/400mL absolute alcohol; 1 g/200mL CHCl2; 1g/40mL benzene [MER06] |
| NIST Chemistry Reference | Phosphorus tetramer(12185-10-3) |
| EPA Substance Registry System | Phosphorus (white or yellow) (12185-10-3) |
Safety information for White phosphorus
Computed Descriptors for White phosphorus
Abamectin manufacturer
New Products
4-Fluorophenylacetic acid (S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate 4-AMINO-2-CHLOROPYRIDINE Benzyl {1-[4-(4- Fluorobenzylcarbamoyl)-5-hydroxy-1- methyl-6-oxo-1,6-dihydropyrimidin-2- yl]-1-methylethyl}-carbamate R,R-Chiraphite 4-BROMO-2-METHYLBENZOIC ACID N-Boc-1,4-butanediamine 2-AMINO-6-METHOXYBENZOTHIAZOLE Cyaclopentane carboxylic acid 3-Nitropyrazole 4-Pyrazolecarboxylic acid 1-Nitropyrazole 2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1- buten-1-yl]-, (3aR,4R,5R,6aS)- 2,5-Dibromopyridine 2-Bromo-6-methyl-4-nitrobenzenamine 2-Chloro-5-fluoro-3-methylphenol (R)-3-Amino-4-(2,4-difluorophenyl)butanoic acid hydrochlorideRelated products of tetrahydrofuran



You may like
-
 12185-10-3 99%View Details
12185-10-3 99%View Details
12185-10-3 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 3112-85-4 98%View Details
3112-85-4 98%View Details
3112-85-4 -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8 -
 3-amino-3-phenylpropanoic acid 98%View Details
3-amino-3-phenylpropanoic acid 98%View Details
614-19-7 -
 (2,2-diethoxyethyl)methylamine 20677-73-0 98%View Details
(2,2-diethoxyethyl)methylamine 20677-73-0 98%View Details
20677-73-0