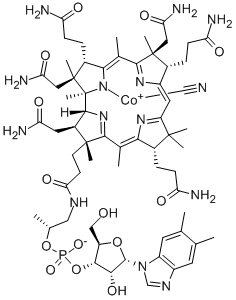
Vitamin B12
Synonym(s):Cyanocobalamin;Vitamin B12;CC;Cyanocobalmin;Cyanocob(III)alamin
- CAS NO.:68-19-9
- Empirical Formula: C63H88CoN14O14P
- Molecular Weight: 1355.37
- MDL number: MFCD00151092
- EINECS: 200-680-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
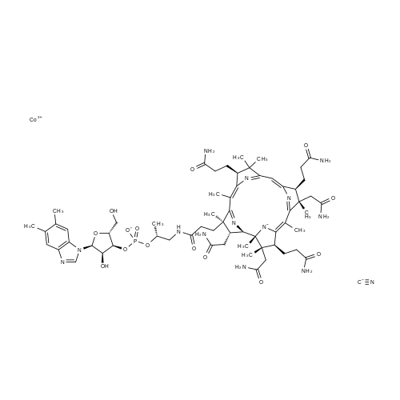
What is Vitamin B12?
Absorption
Vitamin B12 is quickly absorbed from intramuscular (IM) and subcutaneous (SC) sites of injection; with peak plasma concentrations achieved about 1 hour after IM injection .
Orally administered vitamin B12 binds to intrinsic factor (IF) during its transport through the stomach. The separation of Vitamin B12 and IF occurs in the terminal ileum when calcium is present, and vitamin B12 is then absorbed into the gastrointestinal mucosal cells. It is then transported by transcobalamin binding proteins . Passive diffusion through the intestinal wall can occur, however, high doses of vitamin B12 are required in this case (i.e. >1 mg). After the administration of oral doses less than 3 mcg, peak plasma concentrations are not reached for 8 to 12 hours, because the vitamin is temporarily retained in the wall of the lower ileum .
Toxicity
LD50 Oral (mouse): > 5,000 mg/kg .
General toxicity
Vitamin B12 is generally non-toxic, even at higher doses. Mild, transient diarrhea, polycythemia vera, peripheral vascular thrombosis, itching, transitory exanthema, a feeling of swelling of entire body, pulmonary edema and congestive heart failure in early treatment stages, anaphylactic shock and death have been observed after vitamin B12 administration .
Carcinogenesis and mutagenesis
Long term studies in animals examining the carcinogenic potential of any of the vitamin B12 formulations have not completed to date. There is no evidence from long-term use in patients with pernicious anemia that vitamin B12 has carcinogenic potential. Pernicious
anemia is known to be associated with an increased incidence of stomach carcinoma, however, this malignancy has been attributed to the underlying cause of pernicious anemia and has not been found to be related to treatment with vitamin B12 .
Use in pregnancy
No adverse effects have been reported with ingestion of normal daily requirements during pregnancy .
A note on the use of the nasal spray in pregnancy
Although vitamin B12 is an essential vitamin and requirements are increased during pregnancy, it is currently unknown whether the nasal spray form can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. The nasal spray form should be given to a pregnant woman only if clearly needed, as it is considered a pregnancy category C drug in this form. Sufficient well-controlled studies have not been done to this date in pregnant women .
Use in lactation
Vitamin B12 has been found distributed into the milk of nursing women in concentrations similar to the maternal blood vitamin B12 concentrations. No adverse effects have been reported to date with intake of normal required doses during lactation .
Description
Vitamin B12 contains a cobalt atom, a rarity in natural products. The basic vitamin B12 structure has several chemical forms that differ by the substituent on the central cobalt atom. It is a manufactured form of the vitamin made from natural forms and used in supplements because it is highly bioavailable. Vitamin B12 has an extensive history. Hodgkin received the 1964 Nobel Prize in Chemistry for her structure studies on vitamin B12 and other complex molecules. Woodward and Eschenmoser had been awarded earlier Nobels for their achievements in natural product synthesis. Vitamin B12 is found in every cell in the body and has multiple functions, including acting as a cofactor in DNA synthesis. Animal-based foods (meat, milk, and eggs) are the primary sources. Hence, vegetarians, especially vegans, must include plant- or bacterium-derived vitamin B12 supplements in their diets.
Chemical properties
Dark red, crystalline powder or dark red crystals.
Originator
Berubigen,Upjohn,US,1949
History
VITAMIN B12 (Cobalamin), Sometimes also called cyanocobalamin, this vitamin is one of the more recent of the major B complex vitamins to be fully identified, with its structure not definitized (by Hodkin et al.)
The Uses of Vitamin B12
Prototype of the family of naturally occurring cobalt coordination compounds knows as corrinoids. Analogs of vitamin B12 which differ only in the β-ligand of the cobalt are termed cobalamins. Synthesi zed almost exclusively by bacteria. Dietary sources include fish, meat, liver, and dairy products; plants have little or no cobalamins. Converted by the body into its bioactive forms, methylcobalamin and cobamamide, which serve as enzyme cofactors. Severe deficiency may result in megaloblastic anemia and/or neurological impairment.
The Uses of Vitamin B12
vitamin, coenzyme B12
The Uses of Vitamin B12
A coenzyme for synthesis of nucleic acids and metabolism carbohydrates.
The Uses of Vitamin B12
Vitamin B12 is water-soluble required for the normal development of red blood cells. Its deficiency causes pernicious anemia. It is stable in neutral conditions and is more stable for storage than for processing conditions. It is found in meat, fish, and milk.
Background
Cyanocobalamin (commonly known as Vitamin B12) is a highly complex, essential vitamin, owing its name to the fact that it contains the mineral, cobalt. This vitamin is produced naturally by bacteria , and is necessary for DNA synthesis and cellular energy production. Vitamin B12 has many forms, including the cyano-, methyl-, deoxyadenosyl- and hydroxy-cobalamin forms. The cyano form, is the most widely used form in supplements and prescription drugs , . Several pharmaceutical forms of cyanocobalamin have been developed, including the tablet, injection, and nasal spray forms , , . This drug was initially approved by the FDA in 1942 .
Indications
Nasal spray
The cyanocobalamin nasal spray is indicated for the maintenance of vitamin B12 concentrations after normalization with intramuscular vitamin B12 therapy in patients with deficiency of this vitamin who have no nervous system involvement .
Note: CaloMist , the nasal spray form, has not been evaluated for the treatment of newly diagnosed vitamin B12 deficiency.
Injection forms (subcutaneous, intramuscular)
These forms are indicated for vitamin B12 deficiencies due to various causes, with or without neurologic manifestations . Vitamin B12 deficiency is frequently caused by malabsorption, which is often associated with the following conditions :
Addisonian (pernicious) anemia
Gastrointestinal pathology, dysfunction, or surgery, including gluten enteropathy or sprue, small bowel bacterial overgrowth, total or partial gastrectomy
Fish tapeworm infestation
Malignancy of the pancreas or bowel
Folic acid deficiency
Oral forms
Vitamin B12 supplements are widely available and indicated in patients who require supplementation for various reasons. Dose requirements for vitamin B12 which are higher than normal (caused by pregnancy, thyrotoxicosis, hemolytic anemia, hemorrhage, malignancy, hepatic and renal disease) can usually be achieved with oral supplementation . Oral products of vitamin B12 are not recommended in patients with malabsorption, as these forms are primarily absorbed in the gastrointestinal tract .
Production Methods
Vitamin B12 dietary supplements are often prepared commercially by the fermentation of S. griseus, S. aureofaciens, Propionibacterium; or as a by-product of antibiotic production.
Manufacturing Process
The following is taken from US Patent 3,057,851. Milorganite was extracted with water to obtain an aqueous extract containing vitamin B12 active substances. This aqueous extract was purified by treatment with an ion exchange resin according to the following method. An aqueous extract of milorganite, 100 ml containing 300 μg of vitamin B12 active substances and 4.5 grams of total solids, was combined with 0.5 gram of sodium nitrite and 0.4 gram of potassium cyanide. The resulting solution was adjusted to pH 4.0 with hydrochloric acid and heated to boiling. The boiled solution was filtered through a Super-Cel filter surface, and the filter was then washed with water. The filtrate was obtained in a total volume of 130 ml including the washings. Amerlite XE-97, an ion exchange resin of the carboxyl type (Rohm and Haas), was classified to an average wet particle size of 100 to 150 mesh. The classified resin was utilized in the hydrogen form, and was not buffered during the ion exchange fractionation. The classified resin, in the amount of 35 ml, was packed into a glass column having a diameter of 25 mm and a height of 250 mm. The cyanide-treated aqueous extract of milorganite was infused gravitationally into the ion exchange bed at a rate of 3 ml per minute. The effluent was discarded and the resin bed was then washed with the following solutions in the specified sequence: (1) 120 ml of an aqueous 0.1 N hydrochloric acid solution; (2) 75 ml of an aqueous 85% acetone solution; and (3) 70 ml of an aqueous 0.1 N hydrochloric acid solution. After washing, the resin bed was eluted with an aqueous 60% dioxane solution containing 0.1 N of hydrochloric acid. In this elution, 8 ml of colored eluate was collected. This portion of the eluate was found to contain 295 μg of cyanocobalamin and 9 mg of total solids.
brand name
Nascobal (QOL).
Therapeutic Function
Hematinic
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Cyanocobalamin, also known as vitamin B12, belongs to a group of organic compounds important for the normal growth and development of human and animal bodies. It is used for the treatment of trigeminal neuralgia and multiple sclerosis.
Health Hazard
Deficiency diseases or disorders include retarded growth; pernicious anemia; megaloblastic anemia; macrocytic, hyperchromic anemia; glossitis; spinal cord degeneration; and sprue.
Biological Activity
Principal physiological functions include: (1) Coenzyme in nucleic acid, protein, and lipid synthesis; (2) maintains growth; (3) participates in methylations; (4) maintains epithelial cells and nervous system (myelin sheath); (5) erythropoiesis (with folic acid); (6) leukopoiesis.
Biochem/physiol Actions
Vitamin B12 is metabolized to its active form, methylcobalamin. Hindered vitamin B12 uptake is observed in pernicious anemia. Vitamin B12 is essential for erythroblast development. Pregnancy reduces the levels of vitamin B12. Aging reduces vitamin B12 absorption. It works as a coenzyme in fat, carbohydrate and protein metabolism. It is required for growth, genetic stability and survival of cells in vitro. Functions to support one-carbon metabolism. Present in many classical and serum-free formulations.
Safety Profile
Poison by subcutaneous route. Moderately toxic by intraperitoneal route. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of POx and NOx. See also COBALT COMPOUNDS.
Veterinary Drugs and Treatments
Cyanocobalamin is used for treating deficiencies of vitamin B12. Malabsorption of the nutrient secondary to gastrointestinal tract disease, or dietary chromium deficiencies (in ruminants) can be associated with dietary deficiencies of vitamin B12. As there appears to be a high percentage of cats with exocrine pancreatic insufficiency or gastrointestinal disease that are deficient in cobalamin, there is considerable interest in evaluating serum cobalamin (vitamin B12) in these patients. Giant schnauzers may have a genetic defect affecting the location of the cobalamin-intrinsic factor, causing cobalamin deficiency. Dogs with inflammatory bowel disease may also develop cobalamin deficiency.
Biological Functions
Cyanocobalamin corrects vitamin B12 deficiency and improves the symptoms and laboratory abnormalities associated with pernicious anemia (megaloblastic indices, gastrointestinal lesions, and neurologic damage). This drug aids growth, cell reproduction, hematopoiesis, nucleoprotein, and myelin synthesis. It also plays a vital role in fat metabolism, carbohydrate metabolism, and protein synthesis. Cells that undergo rapid division (for example, epithelial cells, bone marrow, and myeloid cells) have a high demand for vitamin B12.
Metabolism
Vitamin B12 or cyanocobalamin obtained from food is initially bound by haptocorrin, a protein found in the saliva with high affinity for B12. This forms a haptocorrin-B12 complex. Cyanocobalamin passes through the stomach and is protected from acid degradation due to its binding to haptocorrin. In the duodenum, pancreatic proteases release cobalamin from the haptocorrin-B12 complex and from other proteins containing protein-bound B12 that have been ingested. Following this, the binding of cobalamin to a second glycoprotein, intrinsic factor, promotes its uptake by terminal ileum mucosal cells by a process called cubilin/AMN receptor-mediated endocytosis. After absorption into enterocytes, intrinsic factor is broken down in the lysosome, and cobalamin is then released into the bloodstream. The transporter ABCC1, found in the basolateral membrane of intestinal epithelial and other cells, exports cobalamin bound to transcobalamin out of the cell . Cyanocobalamin then passes through the portal vein in the liver, and then reaches the systemic circulation. The active forms of cyanocobalamin are methylcobalamin and adenosylcobalamin , .
Purification Methods
Vitamin B12 crystallises from de-ionized H2O, with a solubility in H2O of 1g/80g, and is dried under vacuum over Mg(ClO4)2. The dry red crystals are hygroscopic and can absorb 12% of 2O. A solution at pH 4.5-5 can be autoclaved for 20minutes at 120o without decomposition. Aqueous solutions are stabilised by addition of (NH4)2SO4. [Golding Comprehensive Organic Chem Vol 5 (Ed. Haslam; Pergamon Press, NY, 1979) pp 549584.] Alternatively an aqueous solution of the coenzyme can be concentrated, if necessary in a vacuum at 25o or less, until the concentration is 0.005 to 0.01M (as estimated by the OD at 522nm of an aliquot diluted with 0.01M K-phosphate buffer pH 7.0). If crystals begin to form on the walls of the container, they should be re-dissolved with a little H2O. The concentrated solution is placed in a glass stoppered flask and diluted with 5volumes of Me2CO. After 2-3hours at 3o it is centrifuged (10,000xg/10minutes) in Me2CO-insoluble plastic tubes to remove some amorphous precipitate. The clear supernatant is inoculated with a small crystal of the vitamin and allowed to crystallise overnight at 3o. Crystals are formed on the walls and the bottom of the container. A further 2volumes of Me2CO are added and set aside at 3o to further crystallise. Crystallisation is followed by observing the OD522 of the supernatant. When the OD falls to 0.27, then ca 94% of the crystals have separated. The supernatant is decanted (saved for obtaining a second crop), and the crystals are washed with a little cold 90% aqueous Me2CO (2x), 100% Me2CO (2x), Et2O (2x) at which time the crystals separate from the glass walls. Allow them to settle and remove residual Et2O with a stream of dry N2. The process can be repeated if necessary. The crystals can be dried in air or in a vacuum for 2hours over silica gel at 100o with an 8-9% weight loss. [Barker et al. Biochemical Preparations 10 33 1963.] This material gives a single spot on paper chromatography [see Weissbach et al. J Biol Chem 235 1462 1960.] The vitamin is soluble in H2O (16.4mM at 24o, 6.4mM at 1o), in EtOH and PhOH but insoluble in Me2CO, Et2O, CH2Cl2 and dioxane. UV: max at 260, 375 and 522nm ( 34.7 x 106, 10.9 x 106 and 8.0 x 106 / mole) in H2O. The dry crystals are stable for months in the dark, but aqueous solutions decompose on exposure to VIS or UV light or alkaline CN-, but are stable in the dark at pH 6-7. The vitamin is inactivated by strong acids or alkalies. [Barker et al. J Biol Chem 235 480 1960, see also Vitamin B12 (Zagalak & Friedrich Eds) Walter de Gruyter, Berlin 1979, Beilstein 2 6 IV 3117.]
Properties of Vitamin B12
| Melting point: | >300°C |
| Boiling point: | >300 °C |
| alpha | 23656 -59 ± 9° (dil aq soln) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Sparingly soluble in water and in ethanol (96 per cent), practically insoluble in acetone. The anhydrous substance is very hygroscopic. |
| form | Crystalline Powder or Crystals |
| appearance | dark red crystals or red powder |
| pka | pKa 3.28±0.04(H2O,D2O t=23±0.5 Iunspeci?ed) (Uncertain) |
| color | Red to dark red |
| Odor | dark red cryst. or powd., odorless and tasteless |
| Water Solubility | Soluble |
| Sensitive | Hygroscopic |
| Merck | 14,10014 |
| BRN | 4122889 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Stability: | Hygroscopic. Keep cold and dry. |
| EPA Substance Registry System | Vitamin B12 (68-19-9) |
Safety information for Vitamin B12
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Vitamin B12
| InChIKey | RMRCNWBMXRMIRW-WZHZPDAFSA-L |
Vitamin B12 manufacturer
Omicron Pharmatech Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Vitamin B12 BP/USP 99%View Details
Vitamin B12 BP/USP 99%View Details -
 Vitamin B12 for molecular biology CAS 68-19-9View Details
Vitamin B12 for molecular biology CAS 68-19-9View Details
68-19-9 -
 Cyanocobalamine CAS 68-19-9View Details
Cyanocobalamine CAS 68-19-9View Details
68-19-9 -
 Vitamin B12 for tissue culture CAS 68-19-9View Details
Vitamin B12 for tissue culture CAS 68-19-9View Details
68-19-9 -
 Vitamin B12 95.00% CAS 68-19-9View Details
Vitamin B12 95.00% CAS 68-19-9View Details
68-19-9 -
 Cyanocobalamin PowderView Details
Cyanocobalamin PowderView Details
68-19-9 -
 CyanocobalaminView Details
CyanocobalaminView Details
68-19-9 -
 Vitamin B12 CyanocobalaminView Details
Vitamin B12 CyanocobalaminView Details
68-19-9
