Hydroxocobalamin
Synonym(s):Hydroxocobalamin hydrochloride
- CAS NO.:13422-51-0
- Empirical Formula: C62H90ClCoN13O15P
- Molecular Weight: 1382.82
- MDL number: MFCD09029250
- EINECS: 236-533-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
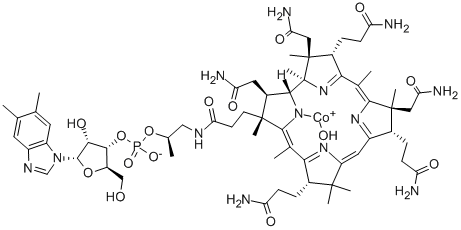
What is Hydroxocobalamin?
Absorption
Readily absorbed from the gastrointestinal tract, except in malabsorption syndromes. Vitamin B12 is absorbed in the lower half of the ileum.
Originator
Alpha-Redisol,MSD,US,1962
The Uses of Hydroxocobalamin
Hydroxocobalamin is an intermediate in the synthesis of Nitritocobalamin(N490240) is a useful synthetic intermediate in the synthesis of Hydroxocobalamin Acetate (H826800); a physiological analog of vitamin B12 where the CN group is replaced with OH. Exists in aqueous solution as an equilibrium mixture of the hydroxy isomer and the ionic aqua isomer (aquacobalamin). Precursor of the coenzymes methylcobalamin and cobamamide. Coordination Compound. Vitamin (hematopoietic).
Indications
For treatment of pernicious anemia and the prevention and treatment of vitamin B12 deficiency arising from alcoholism, malabsorption, tapeworm infestation, celiac, hyperthyroidism, hepatic-biliary tract disease, persistent diarrhea, ileal resection, pancreatic cancer, renal disease, prolonged stress, vegan diets, macrobiotic diets or other restrictive diets. Also for the treatment of known or suspected cyanide poisoning.
Background
Hydroxocobalamin, also known as vitamin B12a and hydroxycobalamin, is an injectable form of vitamin B 12 that has been used therapeutically to treat vitamin B 12 deficiency. It is also used in cyanide poisoning, Leber's optic atrophy, and toxic amblyopia.
Definition
A form of vitamin B 12.
Manufacturing Process
A solution containing 26.3 mg of vitamin B12 in 15 ml of water was shaken
with 78 mg of platinum oxide catalyst and hydrogen gas under substantially
atmospheric pressure at 25°C for 20 hours. Hydrogen was absorbed. During
the absorption of hydrogen the color of the solution changed from red to
brown. The solution was separated from the catalyst and evaporated to
dryness in vacuo. The residue was then dissolved in 1 ml of water and then
diluted with about 6 ml of acetone.
After standing for several hours a small amount of precipitate (about 2 to 3
mg) was formed and was then separated from the solution. This solution was
diluted with an additional 2 ml of acetone and again allowed to stand for
several hours. During this time about 4 to 5 mg of noncrystalline precipitate
formed. This solid was separated from the solution and an additional 2 ml of
acetone was added to the solution. On standing, vitamin B12a began to
crystallize in the form of red needles. After standing for 24 hours, the
crystalline material was separated, yield 12 mg. By further dilution of the
mother liquor with acetone additional crystalline precipitate formed (from US
Patent 2,738,302).
Therapeutic Function
Hematopoietic vitamin
Pharmacokinetics
Hydroxocobalamin is a synthetic, injectable form of Vitamin B12. Hydroxocobalamin is actually a precursor of two cofactors or vitamins (Vitamin B12 and Methylcobalamin) which are involved in various biological systems in man. Vitamin B12 is required for the conversion of methylmalonate to succinate. Deficiency of this enzyme could therefore interfere with the production of lipoprotein in myelin sheath tissue and so give rise to neurological lesions. The second cofactor, Methylcobalamin, is necessary for the conversion of homocysteine to methionine which is essential for the metabolism of folic acid. Deficiency of tetrahydrafolate leads to reduced synthesis of thymidylate resulting in reduced synthesis of DNA which is essential for cell maturation. Vitamin B12 is also concerned in the maintenance of sulphydryl groups in reduced form, deficiency leading to decreased amounts of reduced SH content of erythrocytes and liver cells. Overall, vitamin B12 acts as a coenzyme for various metabolic functions, including fat and carbohydrate metabolism and protein synthesis. It is necessary for growth, cell replication, hematopoiesis, and nucleoprotein as well as myelin synthesis. This is largely due to its effects on metabolism of methionine folic acid, and malonic acid.
Side Effects
Hydroxocobalamin is generally a well-tolerated drug. The adverse effects stem from hypersensitivity reactions to cobalt or other components of the hydroxocobalamin injection. These adverse effects include; exanthema (rash), Itching, fever, nausea, dizziness, rigors, and hot flushes. Anaphylaxis is rare.
Metabolism
Primarily hepatic. Cobalamins are absorbed in the ileum and stored in the liver. They continuously undergo enterohepatic recycling via secretion in the bile. Part of a dose is excreted in the urine, most of it in the first 8 hours.
Properties of Hydroxocobalamin
| Melting point: | >300 °C |
| storage temp. | 2-8°C |
| solubility | methanol: 10 mg/mL at 20 °C, clear, dark red |
| form | neat |
| form | Solid |
| color | Dark Brown to Black |
| Odor | odorless |
| Stability: | Hygroscopic |
| EPA Substance Registry System | Cobinamide, Co-hydroxy-, f-(dihydrogen phosphate), inner salt, 3'-ester with (5,6-dimethyl-1-.alpha.-D-ribofuranosyl-1H-benzimidazole-.kappa.N3) (13422-51-0) |
Safety information for Hydroxocobalamin
Computed Descriptors for Hydroxocobalamin
| InChIKey | YOZNUFWCRFCGIH-ZKJXODNJNA-K |
Hydroxocobalamin manufacturer
Supriya Lifescience Ltd
Venkatasai Life Sciences
Meteoric Biopharmaceuticals Pvt. Ltd.
Deccan Nutraceuticals Pvt. Ltd
Prachem Laboratories
Ralington Pharma
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

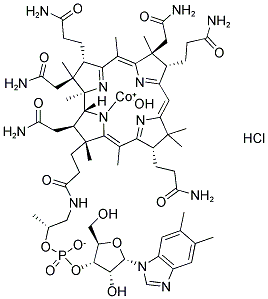

You may like
-
 13422-51-0 98%View Details
13422-51-0 98%View Details
13422-51-0 -
 Hydroxocobalamin 99%View Details
Hydroxocobalamin 99%View Details
13422-51-0 -
 13422-51-0 99%View Details
13422-51-0 99%View Details
13422-51-0 -
 13422-51-0 Hydroxocobalamin 98%View Details
13422-51-0 Hydroxocobalamin 98%View Details
13422-51-0 -
 Hydroxocobalamin 13422-51-0 98%View Details
Hydroxocobalamin 13422-51-0 98%View Details
13422-51-0 -
 13422-51-0 Hydroxocobalamin 98%View Details
13422-51-0 Hydroxocobalamin 98%View Details
13422-51-0 -
 Hydroxocobalamin 99%View Details
Hydroxocobalamin 99%View Details -
 Hydroxocobalamin CAS 13422-51-0View Details
Hydroxocobalamin CAS 13422-51-0View Details
13422-51-0