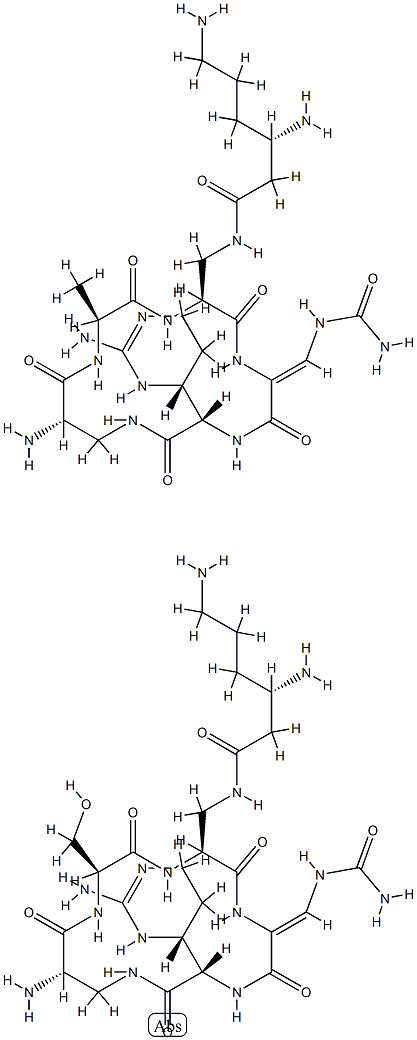
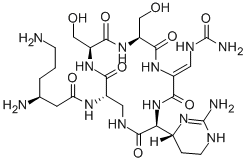
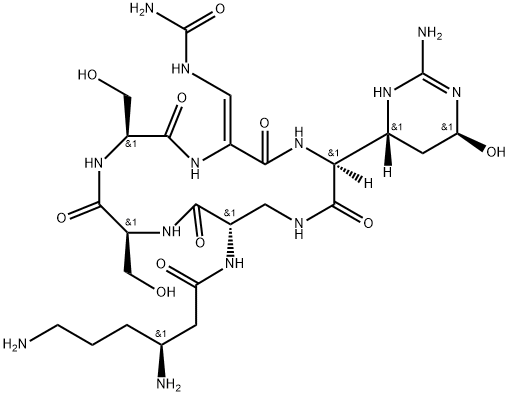
viomycin
- CAS NO.:32988-50-4
- Empirical Formula: C25H43N13O10
- Molecular Weight: 685.7
- MDL number: MFCD01741621
- EINECS: 251-323-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 13:54:39
What is viomycin?
Description
Viomycin was found independently in 1951, in the culture broth of Streptomyces puniceus by Pfizer Research Laboratories and in that of S. floridae by Parke Davis Co. It shows strong activity against Mycobacterium species and moderate activity against gram-positive and gram-negative bacteria. Viomycin has been used by intramuscular administration to treat tuberculosis, but because of its ototoxicity and renal toxicity it is being replaced by more active and less toxic drugs, such as enviomycin and other antituberculotic antibiotics.
Originator
Vinactane,Ciba,US ,1953
The Uses of viomycin
Viomycin is a basic peptide antibiotic, which is an effective agent against multidrug-resistant tuberculosis.
Background
Viomycin is a tuberactinomycin antibiotic that was used to treat Mycobacterium tuberculosis until it was replaced by the less toxic capreomycin. These drugs bind RNA in bacterial ribosomes and inhibit protein synthesis. Viomycin was derived from the actinomycete Streptomyces puniceus.
Indications
Viomycin is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis.
Definition
ChEBI: A cyclic peptide antibiotic produced by the actinomycete Streptomyces puniceus, used in the treatment of tuberculosis.
Indications
Viomycin is a complex polypeptide antibiotic that is active against MDR strains of tuberculosis. Cross-resistance between viomycin and kanamycin is less frequent than between viomycin and capreomycin.
Manufacturing Process
Viomycin is produced by inoculating a nutrient medium with a viable strain of the organism Actinomyces vinaceus. A method for the production of viomycin is set forth in US Patent 2,663,445 comprising inoculating a medium containing soy peptone, beef extract, dextrose, sodium chloride and a silicone antifoaming agent with a spore suspension of Actinomyces vinaceus and incubating the inoculated medium for 120 hours at a temperature of 26°C while passing sterile air through the medium at a rate of 500 ml per liter of medium per minute.
brand name
Viocin Sulfate (Pfizer).
Therapeutic Function
Antitubercular
Biological Activity
Member of the tuberactinomycin family of antibiotics . Inhibits group I intron splicing and prokaryotic protein synthesis. Freezes bacterial ribosomes in either the pre- or post-translational state. Facilitates trans -cleavage of the Neurospora VS ribozyme.
Metabolism
Not Available
Properties of viomycin
| Melting point: | 280 °C |
| Boiling point: | 695.81°C (rough estimate) |
| Density | 1.3613 (rough estimate) |
| refractive index | 1.6700 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Water (Slightly) |
| pka | pKa 8.2 (Uncertain);10.3 (Uncertain) |
| form | Powder |
| Water Solubility | Soluble to 75 mM in water |
| Stability: | Hygroscopic |
Safety information for viomycin
Computed Descriptors for viomycin
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8