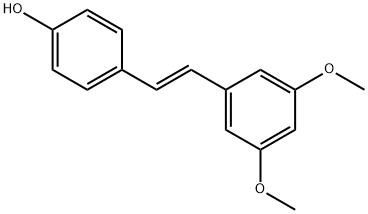
Pterostilbene
Synonym(s):Pterocarpus marsupium;trans-3,5-Dimethoxy-4ʹ-hydroxystilbene;3,5-Dimethoxy-4′-hydroxystilbene;4-[(1E)-2-(3,5-Dimethoxyphenyl)ethenyl]phenol;Pterostilbene, Pterocarpus marsupium - CAS 537-42-8 - Calbiochem
- CAS NO.:537-42-8
- Empirical Formula: C16H16O3
- Molecular Weight: 256.3
- MDL number: MFCD00238710
- EINECS: 611-041-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-06 17:54:05
What is Pterostilbene?
Description
Pterostilbene is a fragrant-smelling hydrocarbon called "styrene", which is a derivative of resveratrol. It is found in blueberries and Pterocarpus marsupium (PM) heartwood. It has anti-inflammatory, anti-thrombotic, anti-cancer, anti-cancer, anti-hyperlipidemia and antibacterial effects.
Chemical properties
Pterostilbene is a white crystalline powder, sensitive to air, soluble in hot methanol, DMSO, insoluble in water. It is a stilbenoid chemically similar to resveratrol and from the leguminous plant Pterocarpus indicus.
Occurrence
Pterostilbene is a natural molecule found in fruits, vegetables and nuts. Blueberries are often quoted as one of the richest sources of pterostilbene. It is also found in almonds,various Vaccinium berries (including blueberries), grape leaves and vines,and Pterocarpus marsupium heartwood.
The Uses of Pterostilbene
Substantial studies demonstrate that pterostilbene has diverse pharmacological activities for the prevention and treatment of diseases including inflammation, cancer, diabetes, and dyslipidemia.
Pterostilbene has been used:
to investigate its anti-oxidative stress activities and the involvement of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-antioxidant response element (ARE) signaling pathway
to determine its effects on transcriptional activation of estrogen receptor-α (ERα) in hormone resistant breast cancer cells)
What are the applications of Application
Pterostilbene, Pterocarpus marsupium is a Cox-1 and Cox-2 inhibitor
Definition
ChEBI: Pterostilbene is a stilbenol that consists of trans-stilbene bearing a hydroxy group at position 4 as well as two methoxy substituents at positions 3' and 5'. It has a role as an antioxidant, an antineoplastic agent, a neurotransmitter, a plant metabolite, an apoptosis inducer, a neuroprotective agent, an anti-inflammatory agent, a radical scavenger and a hypoglycemic agent. It is a stilbenol, a member of methoxybenzenes and a diether. It derives from a hydride of a trans-stilbene.
Preparation
Pterostilbene was synthesized from 3,5-dimethoxybenzyl bromide and p-nitrobenzaldehyde by Witting-Hornor reaction, reduction, diazotization and hydrolysis, with a total yield of 53.9%.
Benefits
Pterostilbene is a naturally-derived stilbenoid structurally related to resveratrol, with potential antioxidant, anti-inflammatory, pro-apoptotic, antineoplastic and cytoprotective activities. Pterostilbene is known to have many pharmacological benefits for the prevention and treatment of a wide variety of diseases, including ( cancer (McCormack and McFadden 2012), dyslipidaemia (Rimando et al. 2005), diabetes (Amarnath Satheesh and Pari 2006), cardiovascular degeneration (Amarnath Satheesh and Pari 2008) and pain (Hougee et al. 2005).
Biological Activity
A cell-permeable methoxylated analog of Resveratrol that displays antioxidant, anti-proliferative, and hypoglycemic properties. Appears to be a better free radical scavenger than Trolox. Moderately inhibits COX-1 & COX-2 activities (IC50 = 19.8 μM & 83.9 μM, respectively) and induces apoptosis in HL60 cells (IC50 = 70 μM). Also prevents DMBA-induced pre-neoplastic lesions (ED50 = 4.8 μM). Reported to decrease plasma glucose levels in streptozotocin-induced diabetic rats comparable to that of Metformin.
Side Effects
The side effects of pterostilbene are relatively minor, but you still need to pay attention to the dosage. Low-dose pterostilbene supplementation improves cholesterol metabolism and reduces triglyceride levels. Side effects include mild weight loss and muscle pain, but dose adjustment is usually not necessary.
References
[1] ESTRELAJOSé M. Pterostilbene: Biomedical applications.[J]. Critical reviews in clinical laboratory sciences, 2013. DOI:10.3109/10408363.2013.805182.
[2] HYUNSOOK KIM Wallace Y Kun Ho Seo. Chemistry of Pterostilbene and Its Metabolic Effects[J]. Journal of Agricultural and Food Chemistry, 2020. DOI:10.1021/acs.jafc.0c00070.
[3] MACICKOVATATIANA. In vivo effect of pinosylvin and pterostilbene in the animal model of adjuvant arthritis.[J]. Neuro endocrinology letters, 2010.
[4] WASAMON NUTAKUL. Inhibitory Effects of Resveratrol and Pterostilbene on Human Colon Cancer Cells: A Side-by-Side Comparison[J]. Journal of Agricultural and Food Chemistry, 2011. DOI:10.1021/jf202846b.
Properties of Pterostilbene
| Melting point: | 89-92 ºC |
| Boiling point: | 420.4±35.0 °C(Predicted) |
| Density | 1.169±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >20mg/mL |
| form | solid |
| pka | 9.96±0.26(Predicted) |
| color | White or off-white |
| Odor | Characteristic |
| λmax | 321nm(MeOH)(lit.) |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 week. |
| InChI | InChI=1S/C16H16O3/c1-18-15-9-13(10-16(11-15)19-2)4-3-12-5-7-14(17)8-6-12/h3-11,17H,1-2H3/b4-3+ |
Safety information for Pterostilbene
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
Computed Descriptors for Pterostilbene
| InChIKey | VLEUZFDZJKSGMX-ONEGZZNKSA-N |
| SMILES | C1(O)=CC=C(/C=C/C2=CC(OC)=CC(OC)=C2)C=C1 |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 Pterostilbene 98% CAS 537-42-8View Details
Pterostilbene 98% CAS 537-42-8View Details
537-42-8 -
 Pterostilbene CAS 537-42-8View Details
Pterostilbene CAS 537-42-8View Details
537-42-8 -
 Pterostilbene 98% (HPLC) CAS 537-42-8View Details
Pterostilbene 98% (HPLC) CAS 537-42-8View Details
537-42-8 -
 Pterostilbene, 97% CAS 537-42-8View Details
Pterostilbene, 97% CAS 537-42-8View Details
537-42-8 -
 Pterostilbene CAS 537-42-8View Details
Pterostilbene CAS 537-42-8View Details
537-42-8 -
 trans-Pterostilbene CAS 537-42-8View Details
trans-Pterostilbene CAS 537-42-8View Details
537-42-8 -
 Pterostilbene, Pterocarpus marsupium CAS 537-42-8View Details
Pterostilbene, Pterocarpus marsupium CAS 537-42-8View Details
537-42-8 -
 Pterostilbene CAS 537-42-8View Details
Pterostilbene CAS 537-42-8View Details
537-42-8