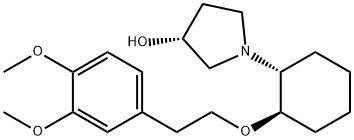
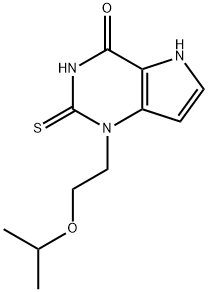
VERNAKALANT HYDROCHLORIDE
- CAS NO.:748810-28-8
- Empirical Formula: C20H32ClNO4
- Molecular Weight: 385.93
- MDL number: MFCD09833303
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
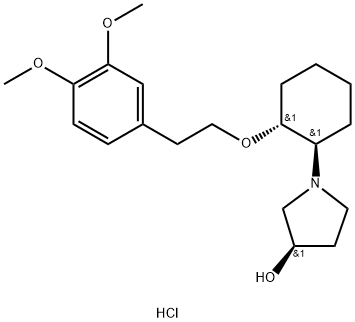
What is VERNAKALANT HYDROCHLORIDE?
The Uses of VERNAKALANT HYDROCHLORIDE
Vernakalant Hydrochloride is a novel, relatively atrial-selective antiarrhythmic drug that effectively and rapidly terminates atrial fibrillation (AF).
The Uses of VERNAKALANT HYDROCHLORIDE
Treatment of patients with atrial fibrillation and atrial flutte.
Clinical Use
Vernakalant is an investigational drug under regulatory review for the acute conversion of atrial fibrillation. The drug was initially developed by Cardiome Pharma under the trade names Kynapid ® and Brinavess ® and its intravenous formulation was further developed by Merck in April 2009. Like other class III antiarrhythmics, vernakalant blocks atrial potassium channels, thereby prolonging repolarization. It differs from typical class III agents by blocking the cardiac transient outward potassium current, with increased potency as the heart rate increases. It also slightly blocks the hERG potassium channel, leading to a prolonged QT interval, which may theoretically increase the risk of ventricular tachycardia.
Synthesis
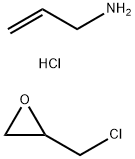
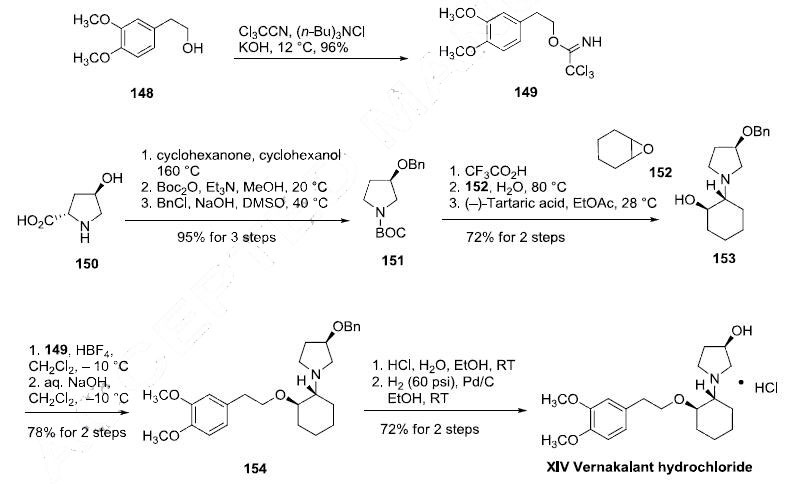
The preparation of vernakalant entails the union of a prolinol derivative 150 with a 3,4-dimethoxyphenethyl alcohol (148) across a cyclohexanyl lynchpin 152 and is described in the scheme. Decarboxylation of commercially available (2S,4R)-4- hydroxyprolinol (150) was effected using cyclohexanol and cyclohexanone at elevated temperatures. Subsequent protection of the nitrogen atom and the oxygen atom within this system resulted in carbamate 151. Acid-mediated removal of the N-protective functionality preceded nucleophilic attack on epoxide 152 in hot water, and the ensuing mixture of diastereomers was separated by classical resolution via the tartrate salt. O-Benzylated vernakalant 154 was obtained when enantiomerically pure alcohol 153 was subjected to trichloroacetimidate 149 (which arose from the corresponding alcohol 148 under modified Williamson conditions. Acidic hydrogenolysis, which the authors report as separate steps, furnished vernakalant hydrochloride (XIV) in excellent overall yield.
Properties of VERNAKALANT HYDROCHLORIDE
| Melting point: | 151-153oC |
| storage temp. | Hygroscopic, Refrigerator, under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Pale Yellow |
Safety information for VERNAKALANT HYDROCHLORIDE
Computed Descriptors for VERNAKALANT HYDROCHLORIDE
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran
You may like
-
 Vernakalant hydrochloride CAS 748810-28-8View Details
Vernakalant hydrochloride CAS 748810-28-8View Details
748810-28-8 -
 Avibactam INT 1View Details
Avibactam INT 1View Details
1416134-48-9 -
 AvibactamView Details
AvibactamView Details
1416134-49-0 -
 Avibactam sodium seriesView Details
Avibactam sodium seriesView Details
1192651-49-2 -
 Avibactam seriesView Details
Avibactam seriesView Details
1192651-80-1 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8