Ferric chloride
Synonym(s):Ferric chloride;Iron(III) chloride;Ferric chloride solution;Iron trichloride;Ferric chloride, Iron trichloride
- CAS NO.:7705-08-0
- Empirical Formula: Cl3Fe
- Molecular Weight: 162.2
- MDL number: MFCD00011005
- EINECS: 231-729-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:52
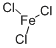
What is Ferric chloride?
Description
Ferric chloride (iron(IH)chloride, FeCl3, CAS No. 7705-08-0) may be prepared from iron and chlorine or from ferric oxide and hydrogen chloride. The pure material occurs as hydroscopic, hexagonal, dark crystals. Ferric chloride hexahydrate (iron(III)chloride hexahydrate, FeCl3*6H2O, CAS No. 10025-77-1) is readily formed when ferric chloride is exposed to moisture.
Chemical properties
Ferric chloride,FeCl3, is a brown crystalline solid and is soluble in water,alcohol,and glycerol. It is also known as anhydrous ferric chloride,ferric trichloride, Flores martis,and iron chloride. Ferric chloride is used as a coagulant for sewage and industrial wastes, as an oxidizing and chlorinating agent,as a disinfectant, in copper etching, and as amordant. In addition, this compound is employed in the ferric chloride test,which is used to assess the relative corrosion resistance of stainless and nickel-base alloys. The ferric chloride test has been shown to be an appropriate measure of the suitability of such alloys for service in paper mill bleach plants and seawater.
Chemical properties
Ferric Chloride is a black-brown, dark-green, or black crystalline solid.
The Uses of Ferric chloride
Ferric Chloride is a nutrient and dietary supplement that serves as a source of iron.
The Uses of Ferric chloride
Ferric chloride, FeCl3, is employed in the ferric chloride test,which is used to assess the relative corrosion resistance of stainless and nickel-base alloys. The ferric chloride test has been shown to be an appropriate measure of the suitability of such alloys for service in paper mill bleach plants and seawater.
In addition, ferric chloride is also a compound that plays a crucial role in the treatment of wastewater. It is a common type of inorganic coagulant that is widely used in both municipal and industrial wastewater treatment processes.
What are the applications of Application
Iron(III) chloride is used for vapor-phase co-reductions with other metal halides
Definition
ChEBI: Iron trichloride is an iron coordination entity. It has a role as a Lewis acid and an astringent.
General Description
Ferric chloride is an orange to brown-black solid. Ferric chloride is slightly soluble in water. Ferric chloride is noncombustible. Ferric chloride is used to treat sewage, industrial waste, to purify water, as an etching agent for engraving circuit boards, and in the manufacture of other chemicals.
Air & Water Reactions
Very hygroscopic. Slightly water soluble, where a 0.1M solution has a pH of 2.0.
Reactivity Profile
Alkali metal hydroxides, acids, anhydrous chlorides of iron, tin, and aluminum, pure oxides of iron and aluminum, and metallic potassium are some of the catalysts that may cause ethylene oxide to rearrange and polymerize, liberating heat, [J. Soc. Chem. Ind. 68:179(1949)]. Explosions occur , although infrequently, from the combination of ethylene oxide and alcohols or mercaptans, [Chem. Eng. News 20:1318(1942)]. Allyl chloride may polymerize violently under conditions involving an acid catalyst, such as sulfuric acid, Ferric chloride, aluminum chloride, Lewis acids, and Ziegler type catalysts (initiators), [Ventrone (1971)].
Hazard
Toxic by ingestion, strong irritant to skin and tissue.
Health Hazard
Inhalation of dust may irritate nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes. Prolonged contact with skin causes irritation and burns.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen chloride fumes may form in fire.
Flammability and Explosibility
Non flammable
Industrial uses
Ferric chloride (FeCl3) is obtained by an iron chlorination method at a temperature of 600–700 °C. Very limited data are available on the use of ferric chloride in the mineral processing industry. Ferric chloride has a depressing effect on barite and can be used in barite–celestite separation. It was also evaluated as a depressant during niobium– zirconium separation. In general, ferric and ferrous compounds are not selective depressants and in many cases are detrimental for flotation of oxidic and industrial minerals as in the case of anionic flotation, fatty acid, iron complexes or oleate iron complexes.
Safety Profile
Poison by ingestion and intravenous routes. Experimental reproductive effects. Corrosive. Probably an eye, skin, and mucous membrane irritant. Mutation data reported. Reacts with water to produce toxic and corrosive fumes. Catalyzes potentially explosive polymerization of ethylene oxide, chlorine + monomers (e.g., styrene). Forms shock sensitive explosive mixtures with some metals (e.g., potassium, sodium). Violent reaction with all$ chloride. When heated to decomposition it emits highly toxic fumes of HCl.
Potential Exposure
Iron chloride is used to treat sewage and industrial waste. It is also used as an etchant for photo engraving and rotogravure; in textiles; photography; as a disinfectant; as a feed additive.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, give milkand get medical attention. Do not induce vomiting. Thesymptoms of metal fume fever may be delayed for 4-12 hfollowing exposure: it may last less than 36 h.Note to physician: Gastric lavage should be performed followed by saline catharsis and anodyne. Administer deferoxamine, I.V. Watch for late stricture. Serum, plasma, orurinary iron levels may be employed to estimate the amountingested.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective in poisoning from iron.
Metabolism
Not Available
Storage
(1) Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.(2) Color Code—Blue: Health Hazard/Poison: Store in asecure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Iron chloride must be stored in tightly closed containers toavoid contact with sulfuric acid, sodium, potassium, allylchloride, and water, since violent reactions occur and toxicvapors may be produced.
Shipping
UN1773 Ferric chloride, anhydrous, Hazard class: 8; Labels: 8-Corrosive material. UN2582 Ferric chlo ride, solution, Hazard class: 8; Labels: 8-Corrosive material
Purification Methods
Sublime it at 200o in an atmosphere of chlorine. It is an “iron-black” coloured powder with green irridescence. Store it in a weighing bottle inside a desiccator as it absorbs moisture from air to form the yellow hexahydrate (see next entry). [Tarr Inorg Synth III 191 1950, Pray Inorg Synth V 153 1957, Epperson Inorg Synth VII 163 1963.]
Structure and conformation
The crystalline solid has a semicovalent layer structure with hexagonal packing of chloride ions, each iron atom being surrounded octahedrally by six chlorines in a BiI3 type structure. The dimers in the vapour phase have a structure similar to that of Al2Cl6 with the iron atoms surrounded by chlorines in a roughly tetrahedral fashion. The magnetic properties of iron(III) chloride in its different environments have been investigated extensively. The magnetic moment at 290°K is 5-73 B.M. and is independent of the field strength. In aqueous hydrochloric acid the room temperature moment is 5-94 B.M. and the hexahydrate has a similar moment (5-95 B.M.).
Incompatibilities
Aqueous solutions are a strong acid. Violent reaction with bases, allyl chloride; sulfuric acid; water. Shock- and friction-sensitive explosive material forms with potassium, sodium and other active metals. Attacks metals when wet.
Waste Disposal
Neutralize with lime or soda ash and bury in an approved landfill.
Properties of Ferric chloride
| Melting point: | 304 °C(lit.) |
| Boiling point: | 316 °C |
| Density | 2,804 g/cm3 |
| vapor density | 5.61 (vs air) |
| vapor pressure | 1 mm Hg ( 194 °C) |
| refractive index | n20/D1.414 |
| Flash point: | 316°C |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | powder |
| color | Yellow |
| Specific Gravity | 2.804 |
| PH | 1 (200g/l, H2O, 20℃) |
| Water Solubility | 920 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,4019 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Stability: | Stable. Very sensitive to moisture. Incompatible with strong oxidizing agents; forms explosive mixtures with sodium, potassium. Hygroscopic. |
| CAS DataBase Reference | 7705-08-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Ferric chloride(7705-08-0) |
| EPA Substance Registry System | Ferric chloride (7705-08-0) |
Safety information for Ferric chloride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H290:Corrosive to Metals H302:Acute toxicity,oral H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Ferric chloride
Ferric chloride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)


You may like
-
 Iron(III) chloride, anhydrous CAS 7705-08-0View Details
Iron(III) chloride, anhydrous CAS 7705-08-0View Details
7705-08-0 -
 Iron(III) chloride, anhydrous CAS 7705-08-0View Details
Iron(III) chloride, anhydrous CAS 7705-08-0View Details
7705-08-0 -
 Iron(III) chloride, anhydrous CAS 7705-08-0View Details
Iron(III) chloride, anhydrous CAS 7705-08-0View Details
7705-08-0 -
 Iron(III) chloride, anhydrous CAS 7705-08-0View Details
Iron(III) chloride, anhydrous CAS 7705-08-0View Details
7705-08-0 -
 Iron(III) chloride, anhydrous CAS 7705-08-0View Details
Iron(III) chloride, anhydrous CAS 7705-08-0View Details
7705-08-0 -
 Ferric Chloride Anhydrous pure CAS 7705-08-0View Details
Ferric Chloride Anhydrous pure CAS 7705-08-0View Details
7705-08-0 -
 ferric chloride CASView Details
ferric chloride CASView Details -
 Iron(III) chloride CAS 7705-08-0View Details
Iron(III) chloride CAS 7705-08-0View Details
7705-08-0
