Palladium nitrate
- CAS NO.:10102-05-3
- Empirical Formula: HNO3Pd
- Molecular Weight: 169.43
- MDL number: MFCD00011169
- EINECS: 233-265-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-06-12 13:02:17
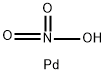
What is Palladium nitrate?
Chemical properties
brown deliquescent crystal(s); heating causes decomposition; used as a catalyst [MER06] [HAW93]
The Uses of Palladium nitrate
Separation of Cl2 and I2; catalyst in organic syntheses.
The Uses of Palladium nitrate
Palladium(II) nitrate is used as a reactant for doping activated carbon for catalysis. It acts as a catalyst for hydrogenation of 1-heptyne and naphthalene. It serves as an important raw material for the preparation of platinum-palladium carbon alloy nanocatalysts for methanol-tolerant oxygen reduction reaction in fuel cells and in the synthesis of copper palladium alloy thin films. Furthermore, it is used in the synthesis of di-phenyl sulfide-modified palladium-titanium dioxide catalysts for acetylene hydrogenation.
The Uses of Palladium nitrate
Palladium matrix modifier may be used in determination of total arsenic by in situ oxidation of diluted urine using graphite furnace atomic absorption spectrophotometer.
What are the applications of Application
Palladium(II) nitrate, Pd 39% min is a strongly oxidizing solid
General Description
Matrix modifier in general, is a buffer that is similar to the chemical environment of aqueous standards and samples. It can also make analyte more thermally stable salts, oxides or metal compounds. It also reduces the volatility of analyte.
Flammability and Explosibility
Not classified
Properties of Palladium nitrate
| Density | 1.118 g/mL at 25 °C |
| solubility | Soluble in dilute nitric acid. |
| form | Crystals |
| color | Red-brown |
| Specific Gravity | 1.118 |
| Water Solubility | Soluble in water, nitric acid. |
| Sensitive | Hygroscopic |
| Merck | 13,7060 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| CAS DataBase Reference | 10102-05-3(CAS DataBase Reference) |
| EPA Substance Registry System | Nitric acid, palladium(2+) salt (10102-05-3) |
Safety information for Palladium nitrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H271:Oxidising liquids;Oxidising solids H290:Corrosive to Metals H314:Skin corrosion/irritation H332:Acute toxicity,inhalation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Palladium nitrate
| InChIKey | GPNDARIEYHPYAY-UHFFFAOYSA-N |
Palladium nitrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Palladium Nitrate 98%View Details
Palladium Nitrate 98%View Details -
 Palladium(II) nitrate 98%View Details
Palladium(II) nitrate 98%View Details -
 Palladium(II) nitrate 99%View Details
Palladium(II) nitrate 99%View Details -
 Palladium nitrate 98%View Details
Palladium nitrate 98%View Details -
 Palladium nitrate CASView Details
Palladium nitrate CASView Details -
 Palladium nitrate CAS 10102-05-3View Details
Palladium nitrate CAS 10102-05-3View Details
10102-05-3 -
 Palladium(II) nitrate solution CASView Details
Palladium(II) nitrate solution CASView Details -
 Palladium Nitrate SolutionView Details
Palladium Nitrate SolutionView Details
10102-05-3
