trofosfamide
- CAS NO.:22089-22-1
- Empirical Formula: C9H18Cl3N2O2P
- Molecular Weight: 323.58
- EINECS: 244-770-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
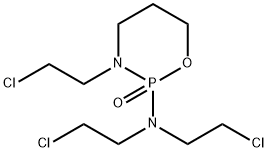
What is trofosfamide ?
Chemical properties
Off-White Low Melting Solid
Originator
Ixoten,Asta,W. Germany,1973
The Uses of trofosfamide
Antineoplastic; one derivative
What are the applications of Application
Trofosfamide is an anti-neoplastic derivative of cyclophosphamide
Definition
ChEBI: Trofosfamide is a member of ifosfamides.
Manufacturing Process
259 g (1 mol) of N,N-bis-(2-chloroethyl)-phosphoric acid amide dichloride,
209 g (1.2 mols) of N-(2-chloroethyl)-N-(3-hydroxypropyl)-amine
hydrochloride (crude), 1,000 cc of ethylene dichloride and 344 g (3.4 mols) of
triethylamine are the reactants. N,N-bis-(2-chloroethyl)-phosphoric acid amide
dichloride is dissolved in the methylene dichloride. N-(2-chloroethyl)-N-(3-
hydroxypropyl)-amine hydrochloride is suspended in this solution and
triethylamine is added thereto dropwise with stirring. The temperature of the
solution rises to boiling, After the termination of the addition, the mixture is
heated to boiling for another 6 hours. Thereafter, the reaction mixture is
cooled down and allowed to stand overnight at about 0°C. The precipitated
triethylamine hydrochloride is filtered off with suction. The resulting solution is
evaporated, the residue (about 370 g) is triturated with about 3.2 liters of
ether and is heated to boiling for a short period of time.
The ethereal solution is decanted from the insolubles (about 90 g). The
solution is rendered to pH 6.5 to 7 by the addition of ethereal hydrochloric
acid and then is filtered over charcoal and thereafter is evaporated. During
evaporation, the temperature should not rise above 40°C. The residue is
dissolved in ether and in an amount corresponding to half of its weight (240 g
of residue, dissolved in 120 cc of ether), the ethereal solution is cooled to -
5°C and is inoculated. After standing for 25 hours, 140 g have been separated
by crystallization. After separation by filtration with suction, the mother liquor
is diluted with ether to 5 times its volume, the solution is filtered over
charcoal, is again evaporated and the residue is again dissolved in a volume
corresponding to half of the weight of the residue. Another cooling to -5°C
and inoculation produces further 18 g of the desired compound. MP: 50° to
51°C. Total yield: 161 g (50% of the theoretical)
Therapeutic Function
Cancer chemotherapy
Safety Profile
Poison by intraperitoneal, subcutaneous, and intravenous routes. Moderately toxic by ingestion. Human mutation data reported. Human systemic effects by unspecified routes: hematuria, leukopenia, and thrombocytopenia. When heated to decomposition it emits very toxic fumes of Cl-, NOx, and POx.
Properties of trofosfamide
| Melting point: | 47-49°C |
| Boiling point: | 104°C |
| alpha | D25 -28.6° (c = 2 in CH3OH) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMF: 50mg/mL; DMSO: 30mg/mL; Ethanol: 50mg/mL; PBS (pH 7.2): 10mg/mL |
| form | A crystalline solid |
| pka | -0.46±0.20(Predicted) |
Safety information for trofosfamide
Computed Descriptors for trofosfamide
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1