OXYPHENYL BUTAZONE
Synonym(s):4-Butyl-1-(4-hydroxyphenyl)-2-phenyl-3,5-pyrazolidinedione;p-Hydroxyphenylbutazone;p-Oxyphenylbutazone;
- CAS NO.:129-20-4
- Empirical Formula: C19H20N2O3
- Molecular Weight: 324.37
- MDL number: MFCD00057278
- EINECS: 204-936-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
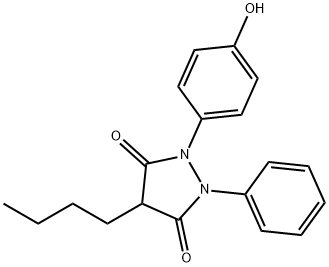
What is OXYPHENYL BUTAZONE?
Chemical properties
Light Beige Solid
Originator
Tanderil,Geigy,UK,1960
The Uses of OXYPHENYL BUTAZONE
Anti-inflammatory;Cyclooxygenase inhibito
Background
Oxyphenbutazone was withdrawn from the Canadian market in March 1985 due to concerns regarding bone marrow suppression.
What are the applications of Application
Oxyphenbutazone is a non-steroidal anti-inflammatory agent
Definition
ChEBI: A metabolite of phenylbutazone obtained by hydroxylation at position 4 of one of the phenyl rings. Commonly used (as its hydrate) to treat pain, swelling and stiffness associated with arthritis and gout, it was withdrawn from the market 1984 following asso iation with blood dyscrasis and Stevens-Johnson syndrome.
Indications
Oxyphenbutazone (Oxalid, Tandearil) is the principal uricosuric metabolite of phenylbutazone. It has the same indications and toxicities as phenylbutazone.
Manufacturing Process
43.2 parts of n-butyl malonic acid ethyl ester are added to a solution of 4.6 parts of sodium in 92 parts by volume of absolute alcohol. 39 parts of pbenzyloxy hydrazobenzene (MP 88° to 90°C) are added. About two-thirds of the alcohol is distilled off and 92 parts by volume of absolute xylene are added. Without removing the sloping condenser, the mixture is stirred for 12 hours at a bath temperature of 140° to 145°C. It is then cooled to 0° to 5°C, 100 parts of ice are added, the xylene is removed, the aqueous solution is extracted twice with chloroform and made acid to Congo red at 0° to 5°C with 6 N hydrochloric acid.
The precipitate is taken up in chloroform, the solution obtained is washed twice with water, then with saturated salt solution, dried over Na2SO4and evaporated under vacuum (bath temperature 20°C). The residue is recrystallized from alcohol and produces 1-(p-benzyloxyphenyl)-2-phenyl-4-nbutyl-3,5-dioxo-pyrazolidine (C) as tiny white needles which melt at 132° to 133°C.
16.6 parts of (C) are suspended in 166 parts by volume of ethyl acetate and, in the presence of 16.6 parts of Raney nickel, hydrogen is allowed to act at room temperature and atmospheric pressure.
After 6 hours the calculated amount of hydrogen has been taken up. The residue obtained after filtering and evaporating is taken up in benzene and extracted twice with diluted sodium carbonate solution. The alkali extract is then made acid to Congo red with 6 N hydrochloric acid and the precipitate is taken up in ethyl acetate. The solution obtained is washed twice with salt solution, dried with sodium sulfate and evaporated. The residue is recrystallized from ether/petroleum ether. 1-(p-hydroxyphenyl)-2-phenyl-4-n
Trade butyl-3,5-dioxo-pyrazolidine melts at 124° to 125°C.
brand name
Tandearil (Novartis);Algi-tandril;Anarreumol-b;Artzone;Buteril;Campozim;Defolgin;Difmedol;Dolo-phlogase;Dolo-tandril;Fibutrox;Gp 40705;Iltazon;Iltoxon;Inflamil;Mindaril;Miyadril;Oflamin;Otone;Oxybutazone;Oxybutol;Oxyperol;Oxyphenbutone;Phlogistol;Phlogont;Phloguran;Pilabutina;Realin;Rheumapax;Segudol;Suganril;Tanal;Tandacot;Teneral;Vefren.
Therapeutic Function
Antiinflammatory
World Health Organization (WHO)
Oxyphenbutazone, a pyrazolone derivative with anti-inflammatory, analgesic and antipyretic activity, was introduced in 1955 for the treatment of rheumatic disorders. It is one of the active metabolites of phenylbutazone and has a similar spectrum of activity including an association with serious and sometimes fatal adverse reactions, notably cases of aplastic anaemia and agranulocytosis. Many national drug regulatory authorities consider that more recently introduced drugs offer a safer alternative for most, if not all, patients requiring antiinflammatory agents. Although oxyphenbutazone has been widely withdrawn it remains available in some countries.
General Description
Oxyphenbutazone is a derivative compound of phenylbutazone. Oxyphenbutazone is one of the metabolites formed in the liver following administration of phenylbutazone. Given orally it causes fewer gastrointestinal adverse effects than phenylbutazone and is used at 300 – 400 mg/d for the same indications as the parent drug.
Biochem/physiol Actions
Oxyphenbutazone is a non-steroid anti inflammatory; anti Mycobacterium tuberculosis agent. Oxyphenbutazone is known to cause inflammatory effects on tissues. Oxyphenbutazone, as a drug, decreases cellular exudates, without involving the pituitary-adrenal axis or the immunity response. Though the drug delivers a number of side effects, it is considered to be less toxic than phenylbutazone, due to decreased rate of intestinal absorption.
Clinical Use
Oxyphenbutazone is a nonsteroidal anti-inflammatory drug, which was used by oral, rectal, or topical administration (400 - 600 mg/d) for the acute treatment of ankylosing spondylitis, chronic polyarthritis, and gout. Because of a high incidence of severe side effects including disturbances of the hematopoietic system like agranulocytosis and aplastic anemia the compound is no longer used. Oxyphenbutazone is a metabolite of phenylbutazone.
Metabolism
Not Available
Properties of OXYPHENYL BUTAZONE
| Melting point: | 109-111°C |
| Boiling point: | 462.71°C (rough estimate) |
| Density | 1.2118 (rough estimate) |
| refractive index | 1.6140 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: soluble10mg/mL, clear |
| form | powder |
| pka | pKa 4.7/10.0±0.2(H2O,t =25,Iundefined) (Uncertain) |
| color | white to brown |
| Water Solubility | 20mg/L(room temperature) |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 129-20-4(CAS DataBase Reference) |
| IARC | 3 (Vol. 13, Sup 7) 1987 |
| EPA Substance Registry System | 3,5-Pyrazolidinedione, 4-butyl-1-(4-hydroxyphenyl)-2-phenyl- (129-20-4) |
Safety information for OXYPHENYL BUTAZONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for OXYPHENYL BUTAZONE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Oxyphenbutazone CAS 129-20-4View Details
Oxyphenbutazone CAS 129-20-4View Details
129-20-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1