Tris(2-carboxyethyl)phosphine hydrochloride
Synonym(s):tris(2-Carboxyethyl)phosphine, HCl;TCEP;TCEP, Hydrochloride - CAS 51805-45-9 - Calbiochem;TCEP, Hydrochloride, Reagent Grade - CAS 51805-45-9 - Calbiochem;TCEP.HCL
- CAS NO.:51805-45-9
- Empirical Formula: C9H15O6P.ClH
- Molecular Weight: 286.65
- MDL number: MFCD00145469
- EINECS: 629-759-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
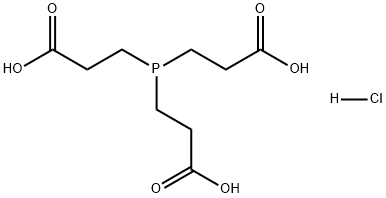
What is Tris(2-carboxyethyl)phosphine hydrochloride?
Description
TCEP HCl (51805-45-9) is a reagent for selective reduction of disulfides in aqueous solutions1-3?with distinct advantages over DTT which are application specific3. For example, spin labels are more stable with TCEP than DTT, Ni2+?(leached from affinity columns) causes rapid oxidation of DTT with no effect on TCEP and for long term storage of proteins TCEP is more stable than DTT with no metal chelator (EGTA) present but DTT is more stable in the presence of metal chelators.3
Chemical properties
White Crystalline Solid
The Uses of Tris(2-carboxyethyl)phosphine hydrochloride
TCEP (Tris(2-carboxyethyl)phosphine hydrochloride) is an alternative which is not pungent, more stable and works even at low pH. It is more selective, i.e. do not reduce metals and buried disulfides.
The Uses of Tris(2-carboxyethyl)phosphine hydrochloride
Biochemical tool for selective reduction of disulfide bridges at low pH; reductant for redox assays.
The Uses of Tris(2-carboxyethyl)phosphine hydrochloride
Reducing agent, specific for disulfidesTris(2-carboxyethyl)phosphine hydrochloride is widely used as a reducing agent to reduce disulfide bonds in proteins and trans-4,5-dihydroxy-1,2-dithiane. It is involved in the reduction of sulfoxides, sulfonyl chlorides, N-oxides, and azides. It plays an important role in the tissue homogenization process for RNA isolation. It acts as a chelating agent and forms complexes with various heavy metal ions such as Zn(II), Cd(II), Pb(II), and Ni(II). Furthermore, it is useful in the labeling of cysteine residues with maleimides.
What are the applications of Application
TCEP, Hydrochloride is a water soluble, non-thiol, strong reducing agent shown to cleave disulfide bonds.
Preparation
The preparation of Tris(2-carboxyethyl)phosphine hydrochloride is as follows:Trimethyl 3,3 ', 3 "-phosphine triphenylpropionate (92.5g, 0.28mol) prepared as described above was added to a 250 ml reaction flask under mechanical stirring conditions and a molar ratio of 12mol/(120ml, 1.44mol), the mixture was heated to 90°C, the solid gradually dissolved, continue to heat the reaction 4h, stirring and cooling with ice water, precipitation of white solid, filter, filter cake with distilled water to get white (2-carboxyethyl) phosphine hydrochloride, dried, weighing 61.3g, yield 82.4%
General Description
Tris (2-carboxyethyl) phosphine (TCEP) is very effective in cleaving disulfide bonds in aqueous solution. It dissolves in water and is odorless, unlike other trialkylphosphines (tributylphosphine). It is also less toxic than 2-mercaptoethanol. These advantages make it better than the other reducing agents.
Reactivity Profile
Nucleophilic Substitution by the Phosphorus Atom of TCEP. 1. The phosphorus atom attacks one sulfur atom along the S-S bond. 2. A thioalkoxyphosphonium cation and a sulfhydryl anion are formed. 3. A rapid hydrolysis releases the second sulfhydryl molecule and the phosphine oxide. There are two main steps in the breaking of disulfide bridges and forming a free sulfhydryl using TCEP: cleavage of the S-S bond; oxidation of the phosphine and release of sulfhydryl.
Biochem/physiol Actions
Tris(2-carboxyethyl)phosphine hydrochloride solution reduces the disulfide bonds and leaves other functional groups intact in proteins.
Storage
Following reconstitution, aliquot and freeze (-20°C). TCEP retains stability when stored at an acidic or alkaline pH; avoid storage at pH 7.0-8.0. Also avoid exposure to high concentrations of phosphate ions (>150 mM). Stock solutions are stable for up to 3 months at -20°C.
References
1) Fischer?et al.?(1993),?In situ reduction suitable for matrix-assisted laser desorption/ionization and liquid secondary ionization using tris(2-carboxyethyl)phosphine; Rapid Commun. Mass Spectrom.,?7?225 2) Kirley?et al.?(1989),?Reduction and fluorescent labeling of cyst(e)ine-containing proteins for subsequent structural analysis; Anal. Biochem.,?180?231 3) Getz?et al. (1999),?A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry; Anal. Biochem.,?273?73
Properties of Tris(2-carboxyethyl)phosphine hydrochloride
| Melting point: | 177 °C |
| Density | 1.041 g/mL at 25 °C |
| refractive index | n |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| pka | 7.66(at 25℃) |
| color | white |
| PH | 7.0(aqueous solution; pH was adjusted with ammonium hydroxide) |
| Water Solubility | Soluble in water and methanol. |
| Sensitive | Moisture Sensitive |
| λmax | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.03 |
| Merck | 14,9085 |
| BRN | 3724376 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in distilled water may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 51805-45-9(CAS DataBase Reference) |
Safety information for Tris(2-carboxyethyl)phosphine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tris(2-carboxyethyl)phosphine hydrochloride
| InChIKey | PBVAJRFEEOIAGW-UHFFFAOYSA-N |
New Products
Boc-N-Me-Val-OH tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin Gabapentin EP Impurity B Ethyl N-(2- cyanoacetyl)carbamate Magnessium Ascorbate 1-Chloro-4-Methyl-2-Nitrobenzene 1,3-Diethyl-1,3-Diphenylurea 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,4-dihydroxybenzaldehyde 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile Benzethonium Chloride Trenbolone Enanthate Prednisolone acetate Cisplatin Chlorodehydromethyl testosterone Ketoconazole 1,3-Di Iodo Benzene Methyl 2-oxo-2,3-dihydrobenzo[d]oxazole-7-carboxylate 3-Hydroxy-4-nitrobromobenzene 4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 2-Ethyl-1,4-diaminobenzene 2-Ethylhexyl 4-aminobenzoateRelated products of tetrahydrofuran



You may like
-
 Tris(2-carboxyethyl)phosphine hydrochloride CAS 51805-45-9View Details
Tris(2-carboxyethyl)phosphine hydrochloride CAS 51805-45-9View Details
51805-45-9 -
 Tris(2-carboxyethyl)phosphine hydrochloride CAS 51805-45-9View Details
Tris(2-carboxyethyl)phosphine hydrochloride CAS 51805-45-9View Details
51805-45-9 -
 Tris(2-carboxyethyl) Phosphine Hydrochloride (TCEP) extrapure AR CAS 51805-45-9View Details
Tris(2-carboxyethyl) Phosphine Hydrochloride (TCEP) extrapure AR CAS 51805-45-9View Details
51805-45-9 -
 Tris(2-carboxyethyl)phosphine Hydrochloride CAS 51805-45-9View Details
Tris(2-carboxyethyl)phosphine Hydrochloride CAS 51805-45-9View Details
51805-45-9 -
 Tris(2-carboxyethyl)phosphine hydrochloride, 98%+ CAS 51805-45-9View Details
Tris(2-carboxyethyl)phosphine hydrochloride, 98%+ CAS 51805-45-9View Details
51805-45-9 -
 Tris(2-carboxyethyl)phosphine hydrochloride 0.5 M solution CAS 51805-45-9View Details
Tris(2-carboxyethyl)phosphine hydrochloride 0.5 M solution CAS 51805-45-9View Details
51805-45-9 -
 Tris(2-carboxyethyl)phosphine CAS 51805-45-9View Details
Tris(2-carboxyethyl)phosphine CAS 51805-45-9View Details
51805-45-9 -
 Tris(2-carboxyethyl)phosphine hydrochloride 98% (HPLC) CAS 51805-45-9View Details
Tris(2-carboxyethyl)phosphine hydrochloride 98% (HPLC) CAS 51805-45-9View Details
51805-45-9
