Fudosteine
- CAS NO.:13189-98-5
- Empirical Formula: C6H13NO3S
- Molecular Weight: 179.24
- MDL number: MFCD00899873
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
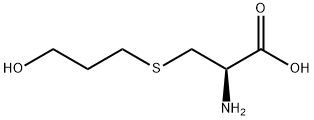
What is Fudosteine?
Description
Fudosteine is a cysteine derivative that interferes with the increase in goblet cell number, expression of the mucin MUC5AC, and subsequent mucin secretion, in airways agonized with tobacco smoke, isoproterenol, lipopolysaccharide, TNF-α, or oxidants. Fudosteine, which is a thiol compound with antioxidant properties, limits NF-κB signaling and reduces inflammatory gene expression. It has greater bioavailability than N-acetyl-cysteine and increases cysteine levels in cells.
Description
Fudostein was launched in Japan as a new mucoactive agent for the treatment of bronchitis and respiratory congestion. This cysteine derivative was obtained from L-cysteine by condensation with either allylic alcohol in the presence of potassium persulfate or the corresponding bromoalcohol in the presence of a base. Fudostein was shown to significantly reduce mucus glycoprotein hypersecretion and inhibit infiltration of airway mucosa by lymphocytes and inflammatory cells in bronchitic rats. When given to bronchitic rabbits, an oral dose of 500 mg/kg daily potently decreased the fucose/ N-acetylneuraminic acid in sputa, so exhibiting mucoregulatory properties. In another study with SO2-exposed rabbits, fudostein suppressed blood flow of tracheal microvasculature increased by SO2, partly due to scavenging of superoxide anion.
Chemical properties
Off-White Powder
Originator
SS Pharmaceutical/Mitsubishi Pharma (Japan)
The Uses of Fudosteine
Fudosteine is a cysteine derivative that interferes with the increase in goblet cell number, expression of the mucin MUC5AC, and subsequent mucin secretion, in airways agonized with tobacco smoke, isoproterenol, lipopolysaccharide, TNF-α, or oxidants. Fudosteine, which is a thiol compound with antioxidant properties, limits NF-κB signaling and reduces inflammatory gene expression. It has greater bioavailability than N-acetyl-cysteine and increases cysteine levels in cells.
The Uses of Fudosteine
An antiinflammatory expectorant MUC5 mucin obstructive pulmonary disease.
The Uses of Fudosteine
Fudosteine is a novel mucoactive agent and a MUC5AC mucin hypersecretion inhibitor. MUC5AC mucin synthesis and the expression of the MUC5AC gene were increased by LPS in rats or TNF-α in NCI-H292 cells; these effects were inhibited by fudosteine treatment
The Uses of Fudosteine
An antiinflammatory expectorant used in the treatment of MUC5 mucin obstructive pulmonary disease.
The Uses of Fudosteine
A demonstrated antiinflammatory
What are the applications of Application
S-(3-Hydroxypropyl)cysteine is a demonstrated antiinflammatory
Definition
ChEBI: Fudosteine is an organic molecular entity.
brand name
Cleanal, Spelear (Mitsubishi Pharma)
Hazard
A reproductive hazard.
Synthesis
Fudosteine is synthesized by condensation of L-cysteine with 3-bromopropyl alcohol in aqueous NaOH, or by condensation of L-cysteine with allyl alcohol by means of aqueous potassium persulfate.
Properties of Fudosteine
| Melting point: | 200-202°C (dec.) |
| Boiling point: | 354.5±42.0 °C(Predicted) |
| Density | 1.301±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Water (Slightly) |
| form | Solid |
| pka | 2.09±0.10(Predicted) |
| color | White to Light Brown |
| CAS DataBase Reference | 13189-98-5(CAS DataBase Reference) |
Safety information for Fudosteine
Computed Descriptors for Fudosteine
Related products of tetrahydrofuran
![L-ALANINE, 3-[(3-HYDROXYPROPYL)SULFINYL]-](https://img.chemicalbook.in/CAS/GIF/209665-22-5.gif)





You may like
-
 Fudosteine 98% CAS 13189-98-5View Details
Fudosteine 98% CAS 13189-98-5View Details
13189-98-5 -
 106-43-4 Para Chloro Toluene (PCT) 99.00%View Details
106-43-4 Para Chloro Toluene (PCT) 99.00%View Details
106-43-4 -
 Pyrrolidine 99.00%View Details
Pyrrolidine 99.00%View Details
123-75-1 -
 88-82-4 2,3,5-Triiodobenzoic Acid 99.00%View Details
88-82-4 2,3,5-Triiodobenzoic Acid 99.00%View Details
88-82-4 -
 Methyl-2-Methoxy-5-Sulfamoyl Benzoate 99.00%View Details
Methyl-2-Methoxy-5-Sulfamoyl Benzoate 99.00%View Details
33045-52-2 -
 532-27-4 2-Chloro Acetophenone 99.00%View Details
532-27-4 2-Chloro Acetophenone 99.00%View Details
532-27-4 -
 Orthochlorobenzaldehyde (2-Chlorobenzaldehyde) 89-98-5 99.00%View Details
Orthochlorobenzaldehyde (2-Chlorobenzaldehyde) 89-98-5 99.00%View Details
89-98-5 -
 12029-98-0 99.00%View Details
12029-98-0 99.00%View Details
12029-98-0