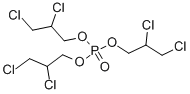
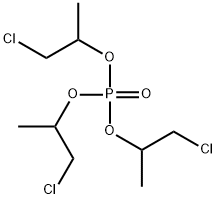
Tris(2-chloroethyl) phosphate
Synonym(s):Phosphoric acid tris(2-chloroethyl) ester
- CAS NO.:115-96-8
- Empirical Formula: C6H12Cl3O4P
- Molecular Weight: 285.49
- MDL number: MFCD00000967
- EINECS: 204-118-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
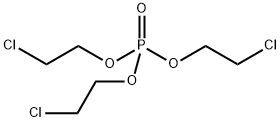
What is Tris(2-chloroethyl) phosphate?
Chemical properties
Clear, transparent liquid. Slightly water soluble. Combustible.
The Uses of Tris(2-chloroethyl) phosphate
Tris(2-chloroethyl) phosphate was used in dynamic air sampling of airborne organophosphate triesters using a solid-phase microextraction device.
The Uses of Tris(2-chloroethyl) phosphate
A human urinary organophosphate flame retardant metabolite.
What are the applications of Application
Tris(2-chloroethyl) phosphate is an organophosphate triester compound used in solid-phase microextractions
Definition
ChEBI: A trialkyl phosphate that is the tris(2-chloroethyl) ester of phosphoric acid.
General Description
Odorless clear liquid. Neutral pH.
Reactivity Profile
Tris(2-chloroethyl) phosphate is incompatible with strong oxidizing agents and strong bases.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data. A skin and eye irritant. Combustible when exposed to heat or flame. When heated to decomposition it emits very toxic fumes of POx and Cl-. See also PHOSPHATES, CHLORIDES, and ESTERS.
Toxicology
The toxicological data of tris(2-chloroethyl) phosphate, LD50 values of 390-1410 mg/kg (and more than 2000 mg/kg in one study), have been reported for rats following oral administration. The substance is nonirritating to very mildly irritating to the skin and mucous membranes. It was not sensitizing to the skin in guinea pigs. Subchronic administration of tris(2- chloroethyl) phosphate caused increased liver weight in rats and mice. Additionally, rats showed increased kidney weights and degenerative changes in the hippocampus region of the brain. Results of mutagenicity studies on tris(2- chloroethyl) phosphate are not consistent, but the majority of tests were negative. Results of carcinogenicity studies suggest that tris(2-chloroethyl) phosphate possesses a weak tumorigenic activity, possibly via an epigenetic mechanism. When pregnant rats were treated with tris(2- chloroethyl) phosphate, toxic effects were found in the mother, but no fetotoxic or teratogenic effects were observed. Impairment of reproduction was observed in a two-generation study in mice.
Properties of Tris(2-chloroethyl) phosphate
| Melting point: | -51 °C |
| Boiling point: | 192 °C/10 mmHg (lit.) |
| Density | 1.39 g/mL at 25 °C (lit.) |
| vapor pressure | <10 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 450 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble7.82g/L |
| form | Liquid |
| color | Clear colorless |
| Water Solubility | 7 g/L (20 ºC) |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 115-96-8(CAS DataBase Reference) |
| IARC | 3 (Vol. 48, 71) 1999 |
| NIST Chemistry Reference | Phosphoric acid, tri-2-chloroethyl ester(115-96-8) |
| EPA Substance Registry System | Tris(2-chloroethyl) phosphate (115-96-8) |
Safety information for Tris(2-chloroethyl) phosphate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H351:Carcinogenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Tris(2-chloroethyl) phosphate
| InChIKey | HQUQLFOMPYWACS-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Tris(2-chloroethyl) Phosphate CAS 115-96-8View Details
Tris(2-chloroethyl) Phosphate CAS 115-96-8View Details
115-96-8 -
 Tris(2-chloroethyl) phosphate CAS 115-96-8View Details
Tris(2-chloroethyl) phosphate CAS 115-96-8View Details
115-96-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
