TRIS hydrochloride
Synonym(s):TRIS HCl;TRIS hydrochloride;Trizma hydrochloride;Tris(hydroxymethyl)aminomethane hydrochloride;tris(Hydroxymethyl)aminomethane, HCl
- CAS NO.:1185-53-1
- Empirical Formula: C4H12ClNO3
- Molecular Weight: 157.6
- MDL number: MFCD00012590
- EINECS: 214-684-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:34
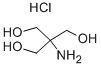
What is TRIS hydrochloride?
Description
Abbreviated as Tris HCl (TRIS hydrochloride), Tris (hydroxymethyl) aminomethane hydrochloride is an organic compound commonly utilized in buffer solutions such as TAE or TBE for electrophoresis gels. It boasts high solubility in water and offers utility within the pH range of 7.0-9.0. The compound is instrumental in preparing Laemmli buffer, one of the most widely used SDS-PAGE buffers, and also finds application in various custom running and loading buffers, frequently in combination with glycine and SDS. Lysis, the initial step of DNA extraction, involves a buffer containing Tris and EDTA (ethylenediaminetetraacetic acid). In this process, EDTA binds to divalent cations such as calcium and magnesium. Due to their role in maintaining cell membrane integrity, their removal by EDTA leads to membrane destabilization. Tris plays a key role as the primary buffering component, maintaining the pH of the buffer at a stable point, typically around 8.0. Additionally, Tris may interact with the LPS (lipopolysaccharide) in the membrane, contributing to further membrane destabilization. This underscores Tris HCl's significance in various laboratory procedures.
Chemical properties
TRIS hydrochloride is a white crystalline powder that has a pKa of 8.08 at 25°C and pH range of 7.0 to 9.0 that coincides with the physiological pH of most living organisms because of which it is commonly used as a component of buffer solutions in biology, biochemistry and molecular biology applications. Tris salts are also used for crystallization of proteins at various pH values. Neither Tris base nor Tris hydrochloride by itself provide adequate buffering capacity. Generally the two need to be mixed together to provide a buffer with pH between 7 and 9 to provide adequate buffering.
The Uses of TRIS hydrochloride
TRIS hydrochloride is a high quality buffer component used in SDS-PAGE. For precise applications, use a carefully calibrated pH meter with a glass/calomel combination electrode. The pH values of all buffers are temperature and concentration dependent. For Tris buffers, pH increases about 0.03 unit per °C decrease in temperature, and decreases 0.03-0.05 unit per ten-fold dilution. It is also used in the robust generation of transgenic mice.
The Uses of TRIS hydrochloride
Tris Hydrochloride is a buffering medium for electrophoresis, molecular biology, and cell culture applications. It may be combined with Tris Base to simplify Tris buffer preparation. Tris Hydrochloride has been incorporated in coelomic fluid and in Cortland medium to store unfertilized rainbow trout eggs. In immunohistochemistry, Tris Hydrochloride has also been used with urea as a method for antigen retrieval.
What are the applications of Application
TRIS hydrochloride is highly soluble in water and is useful in the pH range 7.0-9.0. It is used in the preparation of Laemmli buffer, one of the most common SDS-PAGE buffers. In addition, Tris can also be used for many custom running and loading buffers, most often with glycine and SDS. The use of single-junction pH electrodes that contain silver should be avoided when determining the pH of a solution containing Tris buffer.
Preparation
The preparation of TRIS hydrochloride is as follows:After reacting 2-amino-1,3-propanediol (2.50 g, 27.44 mmol) and formic acid (1.26 g, 27.44 mmol) in 50 ml of water at room temperature for 3 hours, water was distilled off under reduced pressure, A liquid was obtained. By washing the obtained liquid,An ammonium formate salt (3.76 g, 27.44 mmol) was obtained as a colorless transparent liquid.
General Description
TRIS hydrochloride is mainly required for preparation of buffer at physiological range of 7.3 to 7.5. The prepared buffer is compatible with biological fluids. It is of importance to laboratories as a standard pH solution.
Purification Methods
Crystallise the salt from 50% EtOH, then from 70% EtOH. TRIS-hydrochloride is also available commercially in a highly pure state. Otherwise, recrystallise it from 50% EtOH, then 70% EtOH, and dry it below 40o to avoid risk of decomposition. [Beilstein 4 H 304.]
Properties of TRIS hydrochloride
| Melting point: | 150-152 °C |
| Boiling point: | 225℃[at 101 325 Pa] |
| Density | 1.05 g/mL at 20 °C |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | room temp |
| solubility | H2O: 4 M at 20 °C, clear, colorless |
| form | crystalline |
| color | clear colorless (40 % (w/w) in H2O) solution |
| PH | 2.5-4.0 (25℃, 4M in H2O) |
| pka | 8.1(at 25℃) |
| PH Range | 7.0 - 9.0 |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.12 λ: 280 nm Amax: 0.08 |
| BRN | 3675235 |
| Stability: | Stable. Incompatible with strong oxidizing agents, bases, moisture. |
| CAS DataBase Reference | 1185-53-1(CAS DataBase Reference) |
| EPA Substance Registry System | 1,3-Propanediol, 2-amino-2-(hydroxymethyl)-, hydrochloride (1185-53-1) |
Safety information for TRIS hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TRIS hydrochloride
| InChIKey | QKNYBSVHEMOAJP-UHFFFAOYSA-N |
TRIS hydrochloride manufacturer
Innovative
Techno Pharmchem
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Tris(hydroxymethyl)aminomethane hydrochloride CAS 1185-53-1View Details
Tris(hydroxymethyl)aminomethane hydrochloride CAS 1185-53-1View Details
1185-53-1 -
 Tris Hydrochloride (Tris HCl) for molecular biology CAS 1185-53-1View Details
Tris Hydrochloride (Tris HCl) for molecular biology CAS 1185-53-1View Details
1185-53-1 -
 Tris Hydrochloride (Tris HCl) extrapure AR CAS 1185-53-1View Details
Tris Hydrochloride (Tris HCl) extrapure AR CAS 1185-53-1View Details
1185-53-1 -
 Tris(Hydroxymethyl)Methylamine Hydrochloride CAS 1185-53-1View Details
Tris(Hydroxymethyl)Methylamine Hydrochloride CAS 1185-53-1View Details
1185-53-1 -
 Tris hydrochloride, GR 99%+ CAS 1185-53-1View Details
Tris hydrochloride, GR 99%+ CAS 1185-53-1View Details
1185-53-1 -
 TE buffer CAS 1185-53-1View Details
TE buffer CAS 1185-53-1View Details
1185-53-1 -
 Powder White Tris Hydrochloride (CAS Number:1185-53-1), Packaging Size: 1 kg / 5 kg / 10 kg / 25 kgView Details
Powder White Tris Hydrochloride (CAS Number:1185-53-1), Packaging Size: 1 kg / 5 kg / 10 kg / 25 kgView Details
1185-53-1 -
 500gm Tris Hydrochloride PowderView Details
500gm Tris Hydrochloride PowderView Details
1185-53-1
