Tricine
Synonym(s):Tricine;Tricine, N-Tris(hydroxymethyl)methylglycine;Tris Buffer substance;Tris(hydroxymethyl)aminomethane Buffer substance;Tris(hydroxymethyl)aminomethane hydrochloride buffer solution
- CAS NO.:5704-04-1
- Empirical Formula: C6H13NO5
- Molecular Weight: 179.17
- MDL number: MFCD00004277
- EINECS: 227-193-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-11 07:14:29
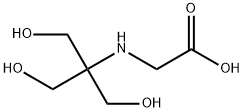
What is Tricine?
Chemical properties
White/clear crystalline powder
The Uses of Tricine
Good's buffers Tricine is used as an electrophoresis buffer and involved in the separation of low molecular weight proteins and peptides. It operates in the pH range between 7.4 and 8.8. It acts as an effective scavenger of hydroxyl radicals during the study of radiation-induced membrane damage. It is also used in resuspension of cell pellets.
A zwitterionic buffer useful in the pH range of 7.4-8.8. Has a pKa 8.05 at 25°C.
A zwitterionic amino acid commonly useful in the pH range of 7.4-8.8. Has a pKa 8.05 at 25°C.
What are the applications of Application
Tricine is a buffer component for separation of low molecular weight peptides
Definition
ChEBI: N-tris(hydroxymethyl)methylglycine is a Good's buffer substance, pKa = 8.15 at 20 ℃. It is functionally related to a member of tris and a glycine. It is a tautomer of a N-tris(hydroxymethyl)methylammonioacetate.
General Description
Tricine and its salts are used as biological buffers to control the pH of body fluids in vitro and in vivo. It acts as a precursor for polymers, oxazolones (with carboxylic acids), and oxazolidines (with aldehydes). Trizma can serve as an alkalizing agent in treating acidosis of the blood.
Biological Activity
Electrophoresis buffer. Working pH range: 7.4 - 8.8. Commonly used in SDS-PAGE to seperate peptides. Also used in other electrophoresis methods, high performance liquid chromatography and ion exchange chromatopgraphy.
Purification Methods
Crystallise Tricine from EtOH and water. [Good et al. Methods Enzymol 24B 53 1968, McGothlin & Jordan Analyt Lett 9 245 1976, Beilstein 18 III/IV 3454.]
Properties of Tricine
| Melting point: | 186-188 °C (dec.) |
| Boiling point: | 311.69°C (rough estimate) |
| Density | 1.05 g/mL at 20 °C |
| vapor pressure | 0.002-0.004Pa at 20-25℃ |
| refractive index | 1.4240 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 4 M at 20 °C, clear, colorless |
| form | crystalline |
| color | clear colorless (40 % (w/w) in H2O) solution |
| Odor | Odorless |
| PH | 4.0-6.0 (1M in H2O) |
| pka | 8.1(at 25℃) |
| PH Range | 7.4 - 8.8 |
| Water Solubility | soluble |
| Merck | 14,9651 |
| BRN | 1937804 |
| Stability: | Stable. |
| CAS DataBase Reference | 5704-04-1(CAS DataBase Reference) |
| EPA Substance Registry System | Glycine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]- (5704-04-1) |
Safety information for Tricine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H320:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P403:Store in a well-ventilated place. |
Computed Descriptors for Tricine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Tricine buffer soln. CAS 5704-04-1View Details
Tricine buffer soln. CAS 5704-04-1View Details
5704-04-1 -
 Tricine CAS 5704-04-1View Details
Tricine CAS 5704-04-1View Details
5704-04-1 -
 Tricine 98% CAS 5704-04-1View Details
Tricine 98% CAS 5704-04-1View Details
5704-04-1 -
 TRICINE Extra Pure CASView Details
TRICINE Extra Pure CASView Details -
![N-[Tris(hydroxymethyl)methyl]glycine CAS 5704-04-1](https://img.chemicalbook.in//Content/image/CP5.jpg) N-[Tris(hydroxymethyl)methyl]glycine CAS 5704-04-1View Details
N-[Tris(hydroxymethyl)methyl]glycine CAS 5704-04-1View Details
5704-04-1 -
 Tricine Cas 5704 04 1View Details
Tricine Cas 5704 04 1View Details
5704-04-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
