Triphosphopyridine nucleotide
Synonym(s):β-NADP;Coenzyme II;NADP;TPN;Triphosphopyridine nucleotide
- CAS NO.:53-59-8
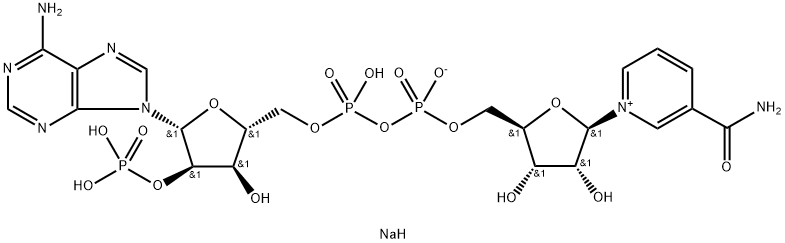
- Empirical Formula: C21H28N7O17P3
- Molecular Weight: 743.41
- MDL number: MFCD00067537
- EINECS: 200-178-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32

What is Triphosphopyridine nucleotide?
Description
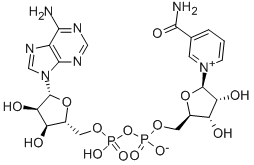
Triphosphopyridine nucleotide is a coenzyme composed of ribosylnicotinamide 5'-phosphate (NMN) coupled by pyrophosphate linkage to the 5'-phosphate adenosine 2',5'-bisphosphate. Triphosphopyridine nucleotide serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH).
Chemical properties
Triphosphopyridine nucleotide is white or off-white powder, it is easy to absorb moisture and deliquescence. pKa{1}=3.9; pKa{2}=6.1. It is soluble in water, methanol, insoluble in ethanol, insoluble in ether and ethyl acetate.
The Uses of Triphosphopyridine nucleotide
β-Nicotinamide adenine dinucleotide phosphate hydrate is suitable for use in:
the measurement of Glucose-6-phosphate dehydrogenase activity
the Cytochrome P450 3A4 assay as a part of NADPH-regenerating system
the Cytochrome P450 2D6 assay as a part of NADPH-regenerating system
the determination of Glucose-6-phosphate content
What are the applications of Application
β-Nicotinamide adenine dinucleotide phosphate is a common form of NADP
Definition
The oxidized form of nicotinamide adenine dinucleotide phosphate (NADP) that receives electrons from photosystem I during photosynthesis. It exists as an anion under normal physiologic conditions.
Biological Functions
Triphosphopyridine nucleotide (NADP) serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH).
Biochem/physiol Actions
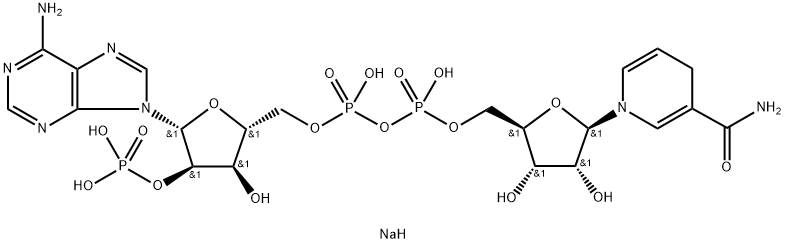
β-Nicotinamide adenine dinucleotide 2′-phosphate (NADP+) and β-Nicotinamide adenine dinucleotide 2′-phosphate, reduced (NADPH) comprise a coenzyme redox pair (NADP+:NADPH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. The NADP+/NADPH redox pair facilitates electron transfer in anabolic reactions such as lipid and cholesterol biosynthesis and fatty acyl chain elongation. The NADP+/NADPH redox pair is used in a variety of antioxidation mechanism where it protects agains reactive oxidation species accumulation. NADPH is generated in vivio by the pentose phosphate pathway (PPP).
Synthesis
Triphosphopyridine nucleotide is prepared by the reaction of NADPH. It is synthesised mainly by the interaction of both NfrA1 enzyme and a Bacillus subtilis under the conditions of bacterial luciferase. Reaction conditions were as follows: with hydrogenchloride; NfrA1 enzyme; nitrofurazone; 2-amino-2-hydroxymethyl-1,3-propanediol In water at 23℃; pH=7.0; Enzyme kinetics; Further Variations; Reagents; Oxidation.
Purification Methods
Purify NMN by passage through a column of Dowex-1 (Clform) and washing with H2O until no absorbance is observed at 260 nm. The tubes containing NMN are pooled, adjusted to pH 5.5-6 and evaporated in vacuo to a small volume. This is adjusted to pH 3 with dilute HNO3 in an ice-bath and treated with 20volumes of Me2CO at 0-5o. The heavy white precipitate is collected by centrifugation at 0o. It is best stored wet and frozen or it can be dried to give a gummy residue. It has max 266nm ( 4,600) and min 249nm ( 3600) at pH 7.0 (i.e. no absorption at 340nm). It can be estimated by reaction with CNor hydrosulfite which form the 4-adducts (equivalent to NADH) which have UV max 340nm ( 6,200). Thus after reaction, an OD340 of one is obtained from a 0.1612mM solution in a 1cm path cuvette. [Plaut & Plaut Biochemical Preparations 5 56 1957, Maplan & Stolzenbach Methods Enzymol 3 899 1957, Kaplan et al. J Am Chem Soc 77 815 1955, Beilstein 22/2 V 168.]
Properties of Triphosphopyridine nucleotide
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: 50 mg/mL, clear, slightly yellow |
| form | powder to crystal |
| pka | pKa1 3.9; pKa2 6.1(at 25℃) |
| color | White to Orange to Green |
| λmax | 260nm(lit.) |
| Merck | 14,6348 |
| EPA Substance Registry System | Adenosine 5'-(trihydrogen diphosphate), 2'-(dihydrogen phosphate), P'.fwdarw.5'-ester with 3-(aminocarbonyl)-1-.beta.-D-ribofuranosylpyridinium, inner salt (53-59-8) |
Safety information for Triphosphopyridine nucleotide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triphosphopyridine nucleotide
| InChIKey | XJLXINKUBYWONI-WFYQBQJJNA-N |
Triphosphopyridine nucleotide manufacturer
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Triphosphopyridine nucleotide 80% CAS 53-59-8View Details
Triphosphopyridine nucleotide 80% CAS 53-59-8View Details
53-59-8 -
 Triphosphopyridine nucleotide 95.00% CAS 53-59-8View Details
Triphosphopyridine nucleotide 95.00% CAS 53-59-8View Details
53-59-8 -
![β-Nicotinamide Adenine Dinucleotide Phosphate [for Biochemical Research] CAS 53-59-8](https://img.chemicalbook.in//Content/image/CP5.jpg) β-Nicotinamide Adenine Dinucleotide Phosphate [for Biochemical Research] CAS 53-59-8View Details
β-Nicotinamide Adenine Dinucleotide Phosphate [for Biochemical Research] CAS 53-59-8View Details
53-59-8 -
 TRIPHOSOPHOPYRIDINE NUCLEOTIDE CAS 53-59-8View Details
TRIPHOSOPHOPYRIDINE NUCLEOTIDE CAS 53-59-8View Details
53-59-8 -
 β-Nicotinamide adenine dinucleotide phosphate hydrate CAS 53-59-8View Details
β-Nicotinamide adenine dinucleotide phosphate hydrate CAS 53-59-8View Details
53-59-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1